Can someone lend me a hand in explaining sig figs involving different units?
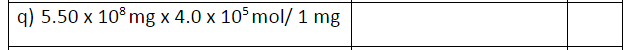
How would I solve and express the answer in sig figs?
How would I solve and express the answer in sig figs?
1 Answer
You multiplied two answers, and one of them has three sig figs. The other has two. So the fewest number of sig figs indicates the number you are less sure about with regards to its precision.
#5.50 xx 10^8 cancel("mg")xx(4.0xx10^5 "mol")/(cancel"1 mg")#
#= 2.2xx10^13 "mols"#
You only get the same number of sig figs from a multiplication or division process as the number you use with the fewest number of sig figs.
You can only be as sure as that which you are least sure about, which is the
In your conversion factor, when you have "per . . . ", that is an exact relationship except for the larger number.
So if you have
Therefore, you have two sig figs, not one.

