Can you control the rate of a reaction by changing the activation energy needed?
1 Answer
Yes.
Explanation:
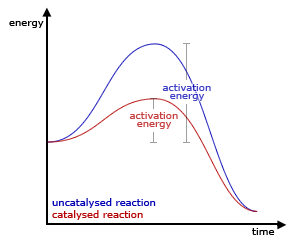
Catalysts lower activation energy so that the reaction will occur faster at lower temperatures, but they are not consumed in the reaction and can be used over and over again.
Enzymes are biological catalysts that lower the activation of all of the chemical reactions in an organism. Without enzymes, a reaction may not occur at all, or require high temperatures incompatible with life, or it may occur at normal body temperature, but without enzymes it might take millions and even billions of years for the reaction to occur, but with enzymes would take place in milliseconds. Amazing, isn't it?
Catalysts in inorganic chemical reactions also lower the activation energy of the reactions. Some catalysts in chemistry are platinum, nickel, and manganese oxide.
http://ch302.cm.utexas.edu/kinetics/catalysts/catalysts-all.php