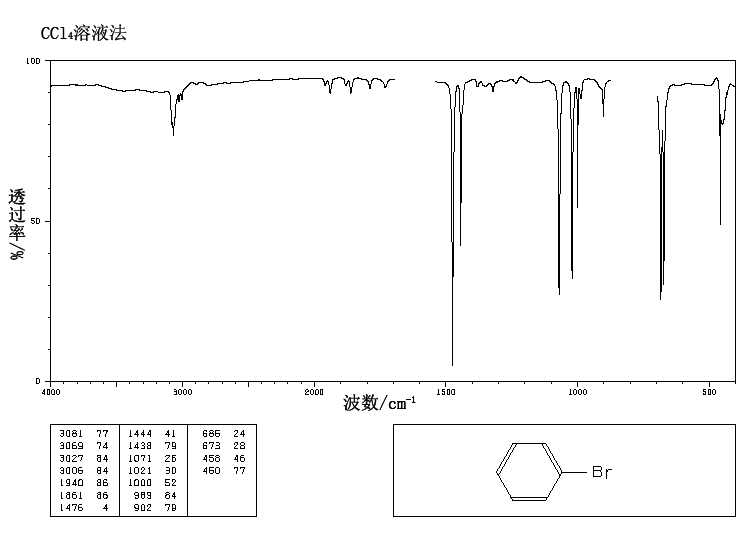
Do halogens appear in the IR spectrum? For example, in a molecule bromine attached to a benzene, where would the bromine appear on the IR spectra?
2 Answers
The
You may not be able to see it if your instrument cuts off at
As an additional answer, if we were specifically talking about, say,
IR-active vibrational motions, i.e. those that change the dipole moment of the molecule, show up in an IR spectrum.
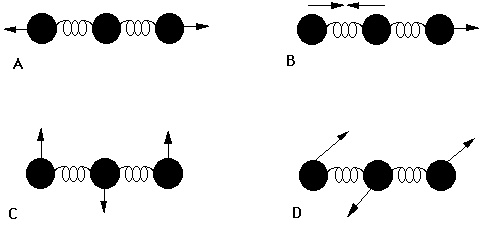
So in the above diagram of
Since diatomic halogens can only stretch one way, and that one way is totally symmetric, there is no change or production of a dipole moment. Thus, there are no IR-active vibrational motions possible.
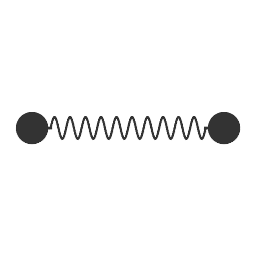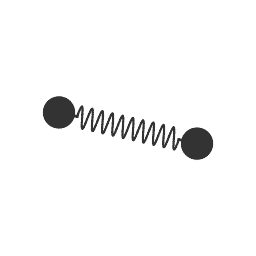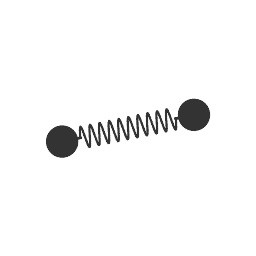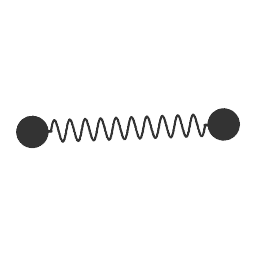
Same with
(Something like


