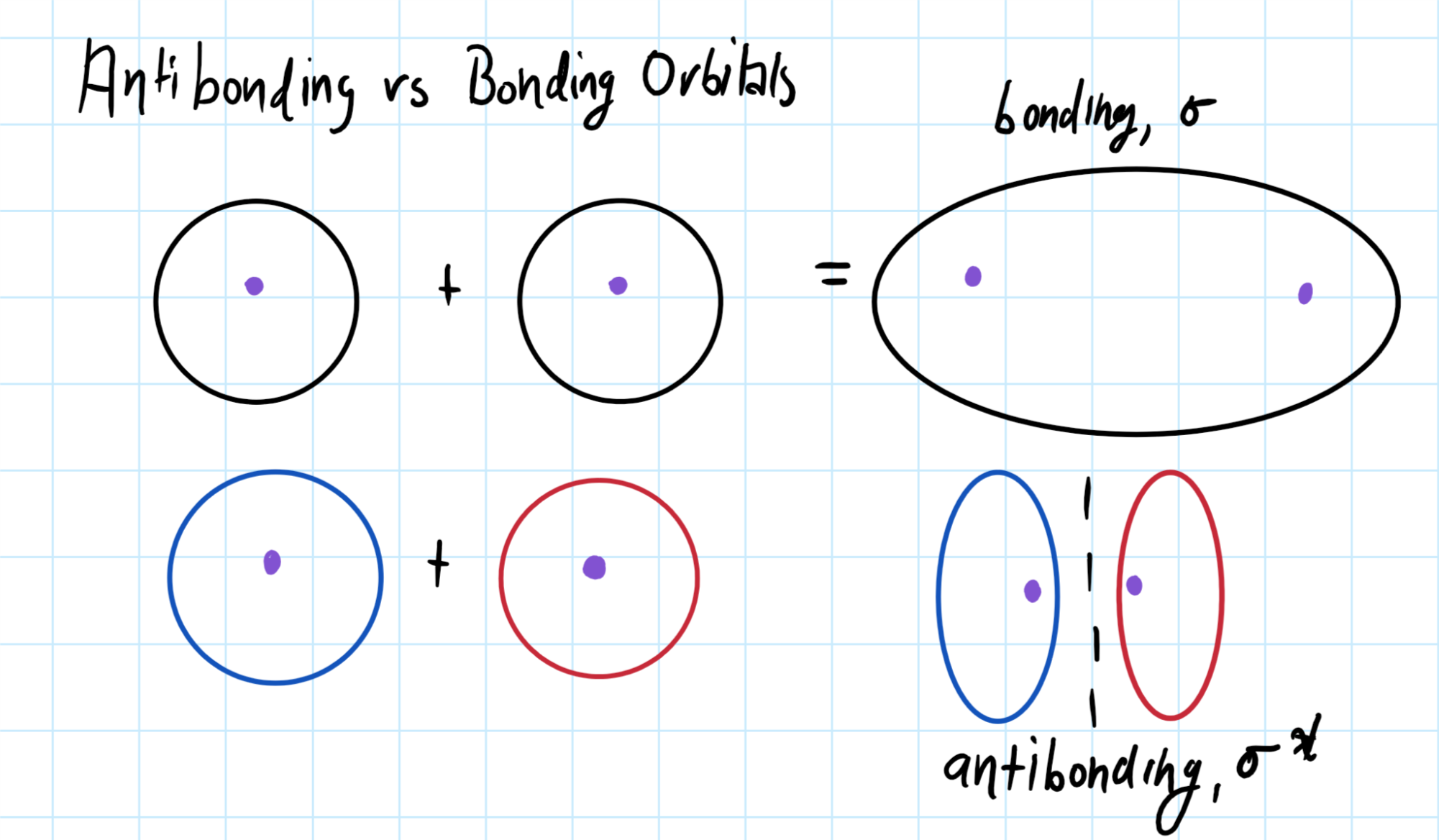
Do sigma bonds overlap?
1 Answer
Dec 5, 2016
Sigma bonds overlap in bonding orbitals and do not overlap in antibonding orbitals, but are formed by the overlap of atomic orbitals.
Explanation:
Consider the molecular orbital manifold shown below: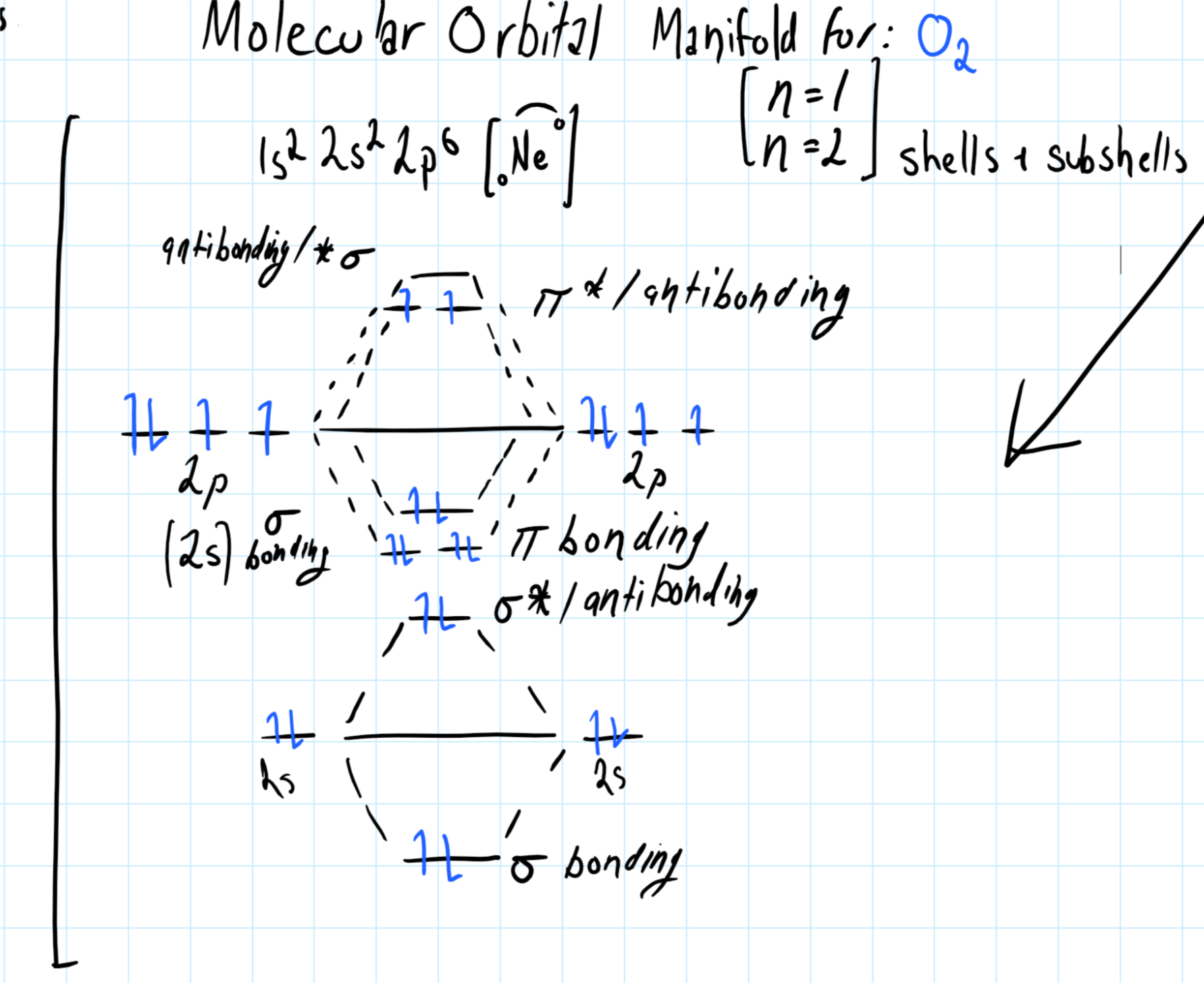
Recall that antibonding orbitals are formed by destructive combination and that bonding orbitals are formed by constructive combination.
For antibonding orbitals, a node forms at the zero and they do not overlap.
As show below: