Do two electrons in one orbital repel each other if they have opposite spin?
1 Answer
Yes.
Explanation:
The electrons will undergo electrostatic repulsion because like charges repel. You may ask "If they're repelling each other, then how can they occupy the same orbital?". Well, that's because the electrons are still being held by the positive nucleus.
This whole idea of electrostatic repulsion explains why Oxygen has a lower first ionization energy than Nitrogen. One would expect Oxygen to have a higher ionization energy since its atomic number is larger than Nitrogen's but this isn't the case.
Observe the following diagrams:

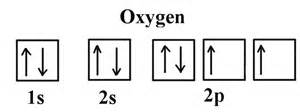
These are the orbital diagrams of oxygen and nitrogen. Notice that Oxygen has paired electrons with an opposite spin in its 2px orbital while Nitrogen has no paired electrons in any of its 2p orbitals.
The paired electrons in the 2px orbital of Oxygen will undergo electrostatic repulsion. This makes it easier for the most loosely held electron (one pointing downwards in this case) to be removed. It's already being repelled by it's fellow electron, so it's not as attracted to the nucleus as the valent electron in Nitrogen, thus, it will not take as much energy to get rid of it as it would for Nitrogen.

