Does the cyanide ion only have one dominant resonance structure? Why?
1 Answer
Yes, you could say that the cyanide ion has only one dominant resonance structure, and here's why you could do that.
You can draw two possible Lewis structures for the cyanide ion,
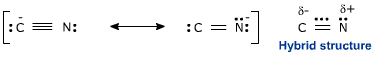
The structure on the left has a triple bond between the two atoms and a lone pair on each atom. The formal charges are zero for nitrogen, since it needs 5 electrons and it gets 5 electrons - 2 from the lone pair and 3 from the three bonds - and (-1) for carbon because it needs 4 electrons and gets 5 - 2 from the lone pair and 1 from each of the three bonds.
The second resonance structure you can draw will have a double bond between the atoms, one lone pair on carbon, and two lone pairs on nitrogen. This time, the (-1) formal charge is on nitrogen, since it now gets 6 electrons - 4 from the lone pairs and 2 from the bonds.
The major resonance contributor will be the first Lewis structure, despite the fact that it has the negative charge placed on the less electronegative atom - carbon. This happens because of the triple bond present, which acts as a powerful stabilizing factor.
The more stable resonance structures have the maximum number of covalent bonds, provided that all atoms have full octets.
Therefore, the major contributor to the hybrid structure shown above is the Lewis structure that has more covalent bonds.

