Does the nitrate ion have one long #N¬O# bond and two short #N¬O# bonds?
1 Answer
Dec 22, 2014
If anything, you might predict that NO₃⁻ has one short N-O bond and two long ones. But all N-O bonds in NO₃⁻ are the same length.
You can draw three equivalent Lewis structures for NO₃⁻. Each has an N=O double bond and two N-O single bonds.
The actual structure is none of these. It is a resonance hybrid of them all.
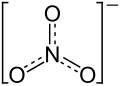
All three bonds have exactly the same length.


