What are the rules for drawing resonance structures?
1 Answer
The rules for drawing resonance structures are:
Explanation:
- You can never move atoms.
- You can move only π electrons or lone pairs that are in
#p# orbitals. - All resonance structures must have the same number of valence electrons.
You can never move atoms
If atoms move, we have isomers, not resonance contributors.
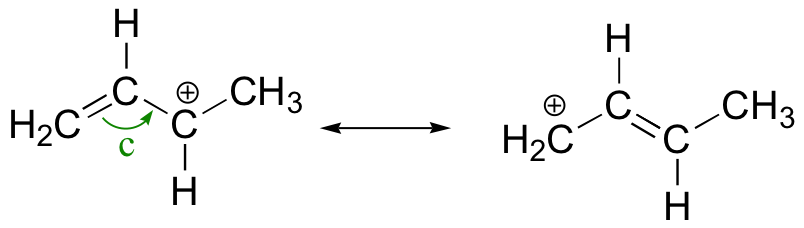
The structures above are resonance contributors, because only electrons have moved. All atoms are in the same position in each structure.
You can move only π electrons or lone pairs that are in
The moving electrons must be on a "donor atom." The "acceptor atom" must be next to the donor atom.
The acceptor atom must have a positive charge or be able to accept an electron pair.
Electrons move towards a positive charge or to a more electronegative atom.

In the first example above, a lone pair of electrons on
In the second example, a pair of π electrons on
In the third example, a pair of π electrons moves onto the more electronegative
All resonance structures must have the same number of valence electrons.
Electrons are not created or destroyed. You must have as many electrons in the structures that you create as there were in the starting structure.
The rule is violated above because structure E has 12 valence electrons and structure F has 14 valence electrons. So E and F are not resonance structures (F also violates the octet rule).

