Explain three exaples of Diastereomers ,enantiomers , mesomers ??? With 3D structures
1 Answer
3D structures will be hard to provide, we can give you two-dimensional pictures...
Explanation:
Now diastereomers are geometric isomers that ARE not mirror images. And a simple example is
Of course enantiomers, are non-superposable mirror-images...
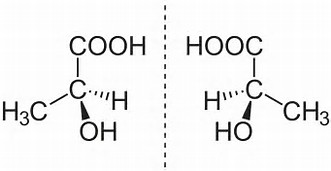 en.wikipedia.org
en.wikipedia.org
And you also want mesomers...which are resonance isomers...and for this we could go back to inorganic chemistry and consider the
And I could write the Lewis structure of
...you can do the same with carbonate or nitrate anions....
Just, apropos of three-dimensional structures, one failing of the modern chemical student is his and her reluctance to use molecular models. These are ALWAYS allowed examination materials, and they seem to be under-utilized....
Given a chiral carbon centre, the interchange of any 2 substituents around that centre gives the enantiomer; interchange again, and it need not be the original 2 substituents, you get the enantiomer of an enantiomer, i.e. the mirror image of a mirror image, i.e. the original isomer. Of course you need to practise how to use them, and you need more practise as to how to represent on the printed page.

