Explain why organic molecules are based on carbon.
1 Answer
By definition, all organic molecule contain carbon. The reason for that is a little trickier to answer, but basically comes down to Carbon having a valence of four (ie Carbon can make four bonds).
This is the way I think of it:
1. Organic molecules as the molecules of life
2. Life is complex
Therefore,
3. Organic molecules need to be complex.
If you look at most inorganic molecules, they a pretty simple. Especially when you compare them to colossal molecules like DNA and polypeptides. To make complex molecules you need to join many parts. Nature does this by making polymers.
Now here's the important part: Carbon is great for making polymers. Having four bonds is perfect for this, because each carbon can link to the carbon before it and after it, and still have two bonds spare for side chains (see picture).
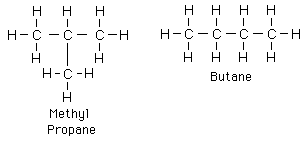
Carbon is the fourth most common element, and the other common elements cannot make polymers (hydrogen, helium, and oxygen). Silicon is also common and can make four bonds, leading to speculation the silicon-based life may exist, though there is no evidence at this time.

