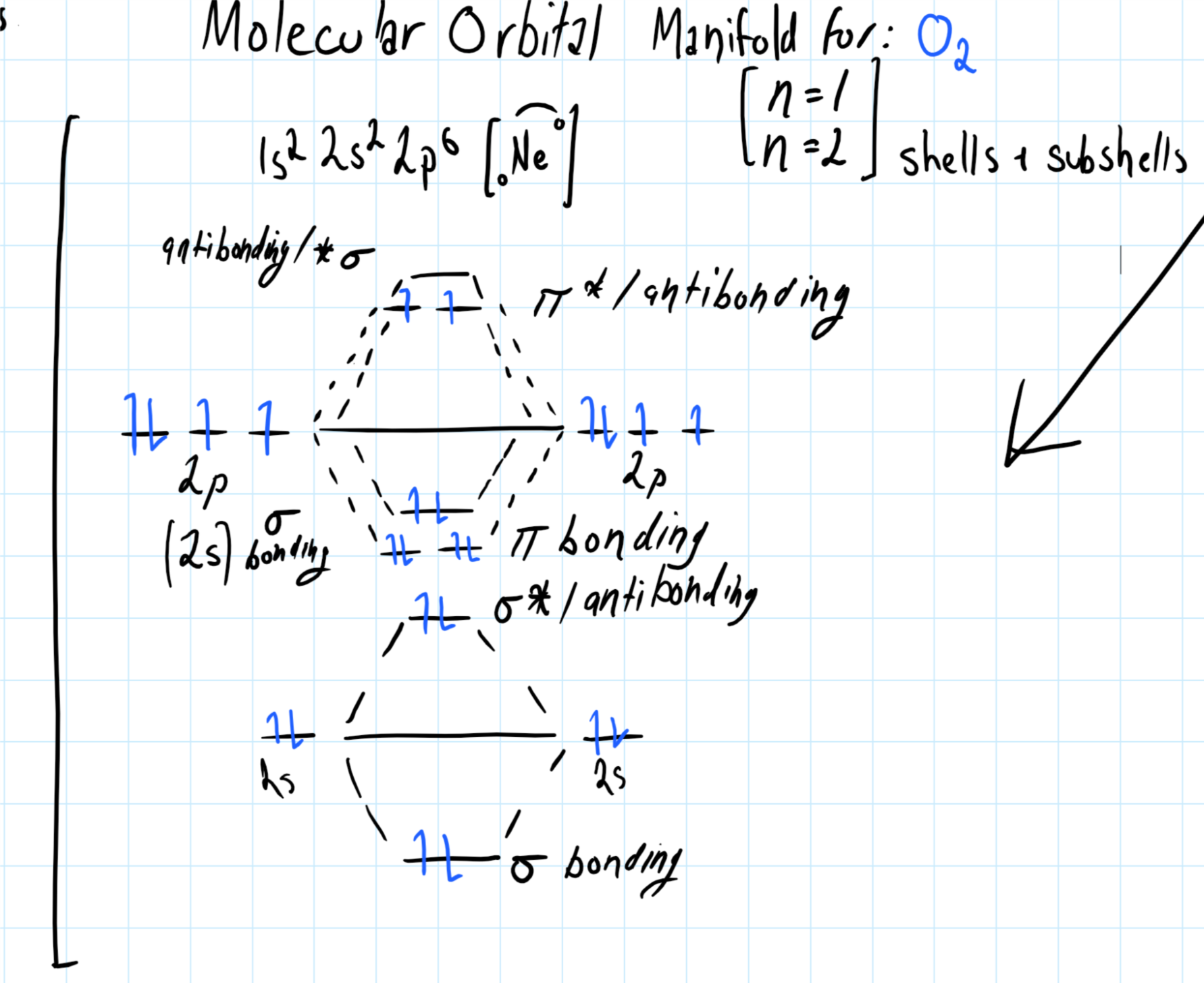
How are sigma and pi bonds form?
1 Answer
Dec 6, 2016
They form from orbital overlap. Sigma bonds will form for single bonds between species and pi bonds form when the bond order is 2 or greater.
Explanation:
For a bonding species, the formation of a
Consider the molecular orbital manifold shown below: