How are sigma bonds drawn?
1 Answer
It depends how you want to draw it. In skeletal formula, it is simply a straight line.
Explanation:
A sigma bond is the first bond between two atoms, and is the strongest sort of covalent bond.
In skeletal formula, used to draw organic molecules with straight lines representing bonds (more than one line means double or triple bonding), a
If you want to draw a sigma bond in terms of orbitals, you do something like this
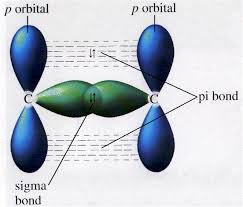
This diagram shows the head-on overlap of two orbitals forming a
The up and down arrows,

