How Can Bonding And Antibonding Electron Can Be Identified In 2p Orbital?
2 Answers
It's a matter of energy
Explanation:
If you have no problem accepting that, for an hydrogen atom, it is the same electron can occupy different orbitals, e.g., 1s, 2p, 4d etc, then you shouldn't have no problem accepting bonding and antibonding electron could be the same electron.
Orbitals in an hydrogen atom have different [quantized] energy levels, the sole electron in the hydrogen can visit any of these orbitals as long as the electron has enough of energy to reach those states.
Because boding is a lower energy than anti-bonding state (of p orbitals or whatever), so the same electron can end up in the anti-bonding state depending on whether it has enough of energy to do so. You break the bonding if you give the electron enough energy, otherwise, it stays in the bonding state.
It cannot be.
Antibonding and bonding electrons can only be assigned when we are talking about molecular orbitals, i.e. within actual molecules. Otherwise, they do not exist.
They are called "bonding" and "antibonding" based on the molecular orbital they belong to.
Consider the molecular orbital (MO) diagram of
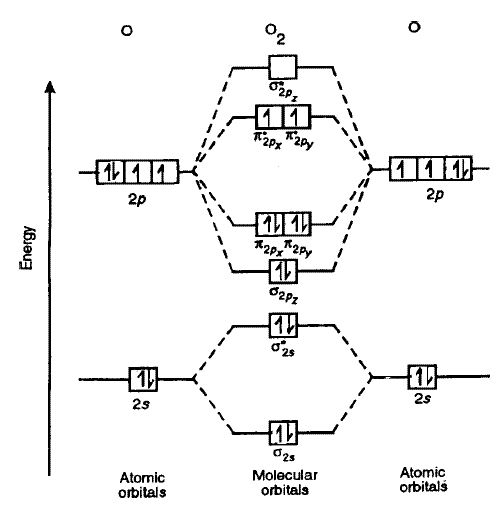
The bonding electrons that are in an MO constructed from the overlap of two
So,
- the
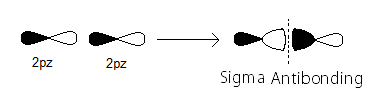
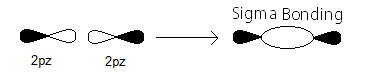
#sigma_(2p_z)# MO contains the#sigma# bonding electrons that were contributed as atomic electrons through the constructive head-on overlap of two#2p_z# atomic orbitals.
There are two such
#sigma_(2p_z)# bonding electrons.
- the
#sigma_(2p_z)^"*"# MO contains the#sigma# antibonding electrons that were contributed atomic electrons through the destructive head-on overlap of two#2p_z# atomic orbitals.
There are zero such
#sigma_(2p_z)^"*"# bonding electrons.
Or, as another example,
- the
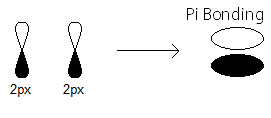
#pi_(2p_x)# MO contains the#pi# bonding electrons that were contributed as atomic electrons through the constructive sidelong overlap of two#2p_x# atomic orbitals.
There are two such
#pi_(2p_x)# bonding electrons.
- the
#pi_(2p_x)^"*"# MO contains the#pi# antibonding electrons that were contributed as atomic electrons through the destructive sidelong overlap of two#2p_x# atomic orbitals.
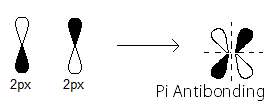
There is one such
#pi_(2p_x)^"*"# antibonding electron.