How can I convert d-talose bond line view to Fischer projection?
1 Answer
A bond-line view gives no stereochemical information, but this is how you convert a wedge-dash formula to a Fischer projection.
Explanation:
The bond-line structure of D-talose is

(from www.chemeddl.org)
It is also the bond-line structure for the 15 other aldohexoses.
The wedge-dash formula gives the stereochemical information.

(from www.chemeddl.org)
In a Fischer projection, The chain is drawn vertically, with the aldehyde group at the top.
The bonds above and below any two adjacent carbon atoms are behind the plane of the paper.
The horizontal bonds are coming out of the paper.
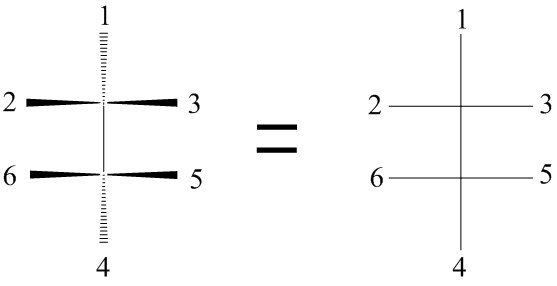
The carbon atoms have a tetrahedral geometry, so the vertical bonds to groups 1 and 4 must be behind the plane of the paper.
When you have a longer vertical chain, you look at any two adjacent atoms.
Those two atoms are in the plane of the paper, and the two atoms directly above and below them are behind the plane of the paper.
You keep the same orientation as you move up and down the chain.

The chain is arch-shaped, as if it you wrapped around a cylindrical tube.
When you flatten the structure onto the surface of the cylinder, you get the Fischer projection of the molecule.
We must view the talose molecule so that each
We must rotate
The
From
The Fischer projection of D-talose is then

(from en.m.wikipedia.org)

