Bond Line View to Fischer Projection
Key Questions
-
Yes, a Fischer projection gives the absolute stereochemistry of a molecule. A bond-line view provides none.
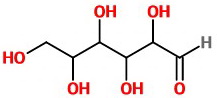
The bond-line structure of glucose is

It tells you only which atom is connected to which.
It gives no stereochemical information.
It could represent any of the 16 stereoisomeric aldohexoses.
The Fischer projection of D-glucose is
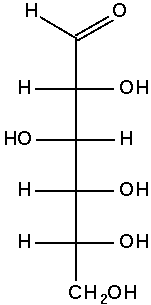
At first glance, this appears to give no more information.
But we must remember that in a Fischer projection the horizontal bonds are coming out of the paper and the vertical bonds are behind the paper.

There is only one molecule that can have this particular combination of wedges and dashes: D-glucose.
Conclusion: A Fischer projection provides more information than a bond-line formula.
-
Answer:
It is useful to convert bond line views to Fischer projections when you want to show the absolute configurations at each chiral centre.
Explanation:
Consider the bond-line structure of D-glucose.

It gives only the connectivity of the atoms. It gives no information on stereochemistry.
There are four chiral centres, so there are
#2^4 = 16# optical isomers. D-glucose is just one of these isomers.To show stereochemistry, we must use wedge-dash formulas.

These formulas give the stereochemical information.
But it is easiest to compare structures using Fischer projections, because they are always written in the same way.
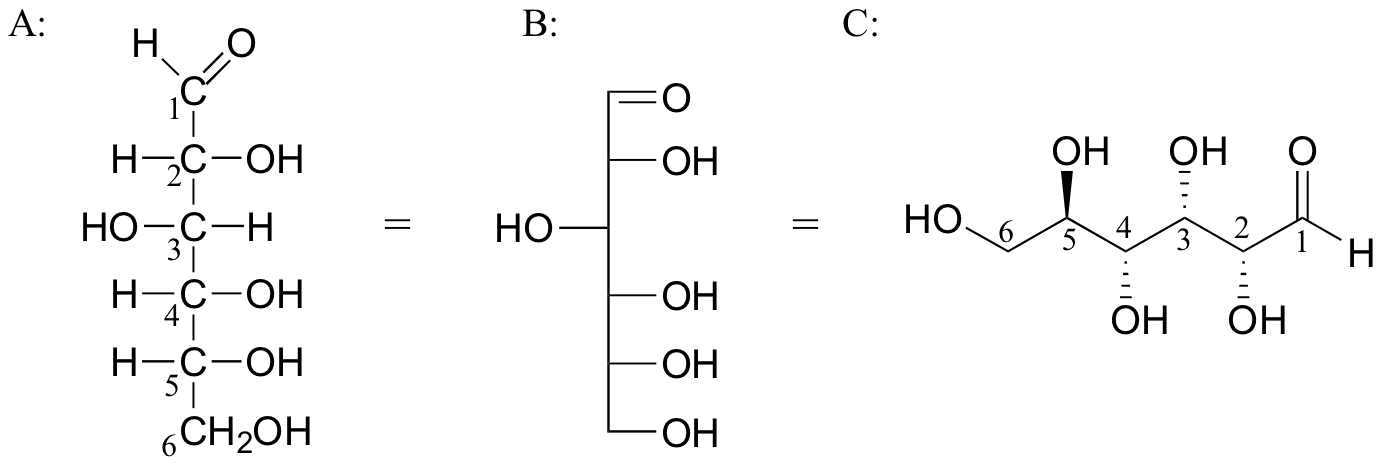
If we make the wedge-dash formula vertical with C-1 at the top, it becomes structure B above. It is then easy to convert B into the Fischer projection A.
Here’s a video on converting line drawings to Fischer projections.
-
Answer:
Here are the steps to convert a bond-line view to a Fischer projection.
Explanation:
The bond-line structure of D-altrose is
This structure gives no stereochemical information.
But here's how to convert a wedge-dash formula to a Fischer projection.
The wedge-dash structure of D-altrose is

(from www.chemeddl.org)Now we can convert the wedge-dash to a Fischer projection.
(a) Arrange the molecule so that the chiral carbons and the longest continuous chain are in a vertical arrangement, with the aldehyde group at the top and the hydroxymethyl group at the bottom. Put crosses at C-2, C-3, C-4, and C-5.
(b) Put the OH group at C-2 on the correct side of the cross.
View the molecule from an angle that puts C-2 closest to our eye and C-1 at the top (the upper left). The OH group is on the left.
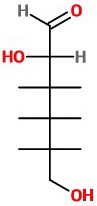
(c) Put the OH group at C-3 on the correct side of the cross.
View the molecule from the lower left. The OH group is on the right.
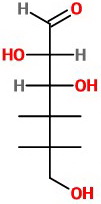
(d) Repeat at C-4, viewing from the upper left.
The OH group is on the right.
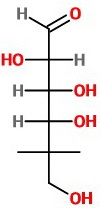
(e) Repeat at C-5, viewing from the lower left.
The OH group is on the right.
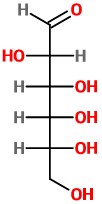
And we have the Fischer projection of D-altrose.