How can I draw the more stable chair conformer of cis -1-ethyl-2-methylcyclohexane?
1 Answer
Start by drawing the wedge-dash notation for cis-1-ethyl-2-methylcyclohexane, which would look something like this
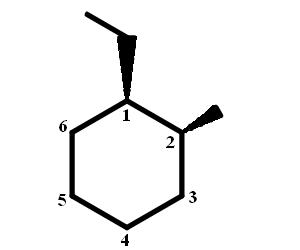
SInce the ethyl and methyl group are in a cis relationship, both must be placed either on wedges, or on dashes. As you can see, this drawing shows them both on wedges.
Now, draw the first chair conformation by placing the ethyl group UP on an axial bond and the methyl group UP on an ecuatorial bond. This will be the placement of the groups because, if you go around the ring, same orientation groups will fall on alternating axial and equatorial bonds.
In order to determine the most stable chair for this molecule, draw the chair flip conformer as well. Start with an empty chair flip template
Now, when performing a chair flip, it is important to remember that orientation does not change - UP will remain UP and DOWN will remain DOWN; however, axial bonds will become equatorial and vice versa.
This means that the ethyl group will go from being UP on an axial bond to being UP on an equatorial bond. The exact same thing will happen with the methyl group, only this time UP equatorial will become UP axial.
Now to determine stability. For cyclohexane chair conformations, stability will depend on what type of bonds (axial or equatorial) the larger groups are placed. Ideally, both methyl and ethyl groups should be on equatorial bonds in order for the chair conformation to be the most stable.
In this case, they alternate between being on axial and on equatorial bonds. As a result, the most stable chair conformer will have the largest group on an equatorial bond, which means the second chair (the flipped one) is the more stable of the two.

