What is a cyclohexane chair flip?
1 Answer
Just to retire this question.....this is really something you should address with a model....
Explanation:
A cyclohexane ring lies ROUGHLY in the plane, and each CARBON bears an AXIAL substituent, and an EQUATORIAL substituent.
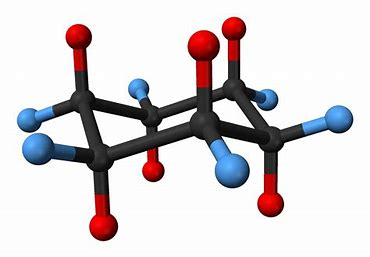
There are SIX blue equatorial substituents, and SIX red axial substituents....and there is a fairly soft energy of exchange which exchanges the axial and equatorial substituents... If the substituent is LARGER than hydrogen, the conformation with the bulky substituent in the equatorial position is the one that is most energetically stable in that it minimizes the interaction between the axial substituents. And again I stress that you should verify what I say and mean with a model in your hands....
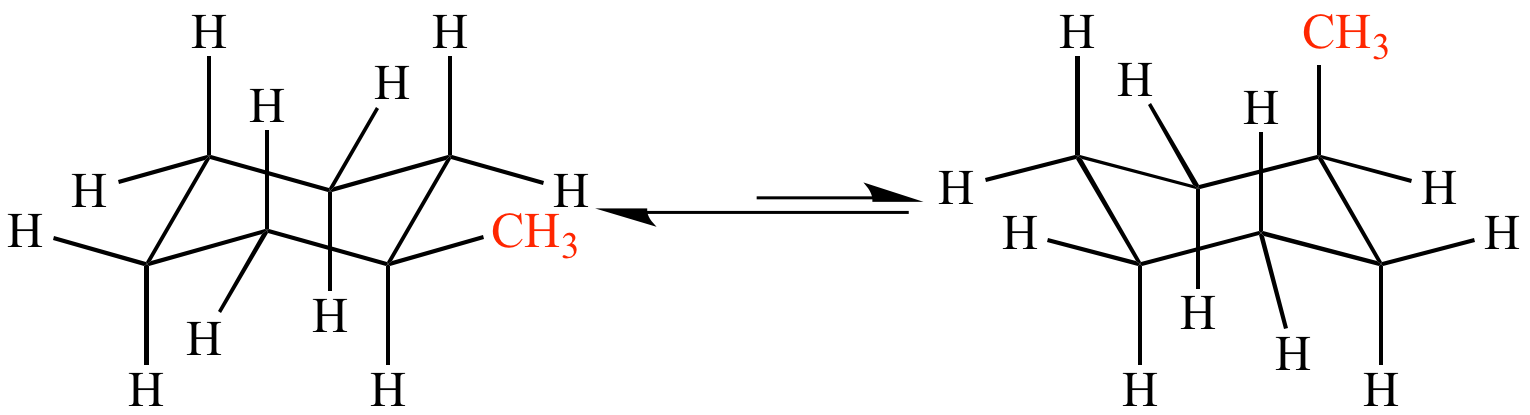
Now when these conformations undergo transition, the axial and equatorial substituents EXCHANGE DIRECTLY.... it is a non-trivial proposition to draw a diagram of the process, and it will repay some practice should you be asked to represent the exchange in an exam...

