How can I draw the more stable chair conformer of trans -1-ethyl-2-methylcyclohexane?
1 Answer
Start by drawing the wedge-dash notation for trans-1-ethyl-2-methylcyclohexane, which looks like this
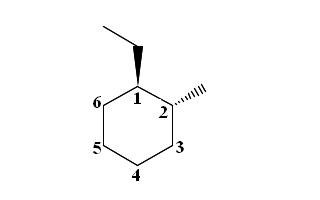
Since you're dealing with a trans molecule, the ethyl group, which is attached to carbon (1), and the methyl group, which is attached to carbon (2), must be on opposite sides of the ring, i.e. they cannot be both on a dash or on a wedge.
For cyclohexane terminology, one must be UP, which is equivalent to being on a wedge, and one must be DOWN, which is equivalent to being on a dash.
Now you draw the first chair conformation for the molecule by placing the ethyl group in UP position on an axial bond and the methyl group in DOWN position on an axial bond as well
Before deciding whether or not this is the most stable chair conformation, you must draw the chair flip conformation as well. Start by drawing an empty chair flip template
Now to place the two groups. Ethyl will be attached to carbon (1) in the UP postion, while methyl will be attached to carbon (2) in the DOWN position. Here's how that would look
Notice that each group's orientation has remained unchanged, but now both groups are on equatorial bonds. Since chair conformers are more stable when larger groups are on equatorial bonds, this will be the most stable of the two.

