How can I draw the structural formulas for all the isomers of C4H7Cl? Are there any enantiomers or diastereomers?
1 Answer
Since there are 18 isomers, you must use a systematic process.
First, if you replace the Cl with H, the formula becomes C₄H₈. An alkane would have the formula C₄H₁₀, so the compound may contain a double bond or a ring.
- Draw all possible C-4 alkenes containing 4 C atoms in a row.
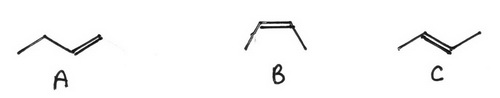
- Draw all possible C-4 alkenes containing 3 C atoms in a row.

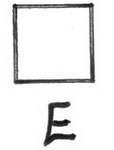
- Draw all possible cyclobutanes
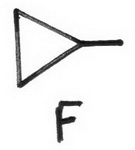
- Draw all possible C-4 cyclopropanes
Now we add the Cl atom.
- Add a Cl atom to all possible locations on A.
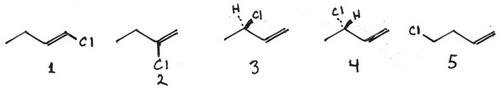
1 1-chlorobut-1-ene
2 2-chlorobut-1-ene
3 (R)-3- chlorobut-1-ene
4 (S)-3- chlorobut-1-ene
5 4-chlorobut-1-ene
3 and 4 are enantiomers.
- Add a Cl atom to all possible locations on B
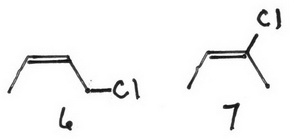
6 (Z)-1-chlorobut-2-ene
7 (E)-2-chlorobut-2-ene
- Add a Cl atom to all possible locations on C.
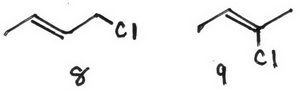
8 (E)-1-chlorobut-2-ene
9 (Z)-2-chlorobut-2-ene
- Add a Cl atom to all possible locations on D.

10 1-chloro-2-methylpropene
11 1-chloro-2-methylpropene
- Add a Cl atom to all possible locations on E.
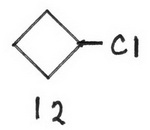
12 chlorocyclobutane
- Add a Cl atom to all possible locations on F.

13 (chloromethyl)cyclopropane
14 1-chloro-1-methylcyclopropane
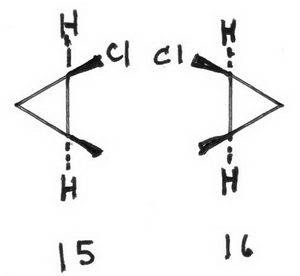
15 (1S,2R)-1-chloro-2-methylcyclopropane
16 (1R,2S)-1-chloro-2-methylcyclopropane
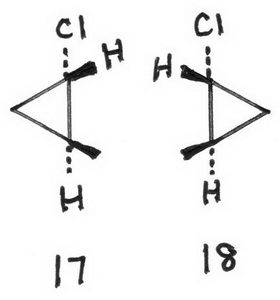
17 (1S,2S)-1-chloro-2-methylcyclopropane
18 (1R,2R)-1-chloro-2-methylcyclopropane
The enantiomeric pairs are 15, 16 and 17, 18.
The diastereomeric pairs are (15, 17), (15, 18), (16, 17), and (16, 18).

