How do you draw the cis and trans isomers for 1-bromo-4-chlorocyclohexane?
1 Answer
Let's start with the wedge-dash structures.
Draw two cyclohexane rings.
On one, put wedges at C-1 and C-4 (cis).
On the other, put a wedge at C-1 and a dash at C4 (trans).
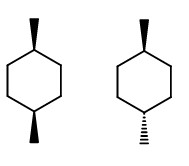
Now, put a Br at C-1 and a Cl at C-4.
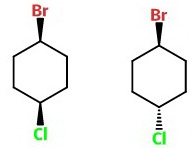
The structure with two wedges is cis-1-bromo-4-chlorocyclobutane.
The structure with a wedge and a dash is trans-1-bromo-4-chlorocyclobutane.
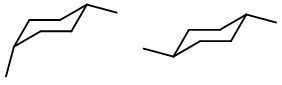
Now, let's draw the chair structures.
Draw two cyclohexane rings.
Since equatorial substituents are more stable, draw one ring with an equatorial bond at C-1 and an axial bond at C-4.
On the other ring, put equatorial bonds at C-1 and C-4.
The bulky Br atom will occupy the equatorial position at C-1 in both structures.
The Cl atom will occupy the bonds at C-4.
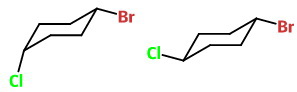
The structure on the left (with an axial Cl) is cis-1-bromo-4-chlorocyclohexane.
The structure on the right (with both bonds equatorial) is trans-1-bromo-4-chlorocyclohexane.

