Of all the possible cyclooctanes that have one chloro substituent and one methyl substituent, which ones do not have any asymmetric centers?
1 Answer
Sep 24, 2015
The only isomers without chiral centres are 1-chloro-1-methylcyclohexane, cis-1-chloro-5-methylcyclohexane, and trans-1-chloro-5-methylcyclohexane.
Explanation:
In order for the centres to be achiral, they must have a plane of symmetry passing through them.
The only possibilities are
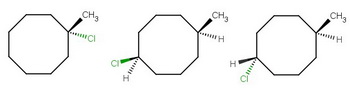 Cyclooctane
Cyclooctane
The first structure, 1-chloro-1-methylcyclohexane, has a vertical plane of symmetry that includes,
The other two structures, cis- and trans-1-chloro-5-methylcyclohexane, have a vertical plane of symmetry that includes

