How can I know the relative number of grams of each substance used or produced with chemical equations?
1 Answer
Aug 26, 2014
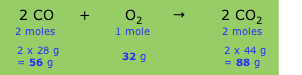
You use the balanced chemical equation and the molar mass of each substance.
Consider the reaction
2C₂H₂ + 5O₂ → 4CO₂+ 2H₂O
Below each substance, write its coefficient and molar mass, like this:
2C₂H₂ + 5O₂ → 4CO₂+ 2H₂O
2×26.04 g + 5×32.00 g → 4×44.01 g + 2×18.02 g
52.08 g + 160.0 g → 176.0 g + 36.04 g
This tells you that 52.08 g of C₂H₂ react with 160.0 g of O₂ to form 176.0 g of CO₂ and 36.04 g of H₂O.
The relative numbers of grams are
C₂H₂ : O₂ : CO₂ : H₂O = 52.08 : 160.0 : 176.0 : 36.04
Here's another example: