How can I read molecular orbital diagram?
1 Answer
WARNING. This is a long answer.
Let's analyze a molecular orbital (MO) diagram for CO.

Points to Note:
-
Individual atomic orbitals (AOs) are on the far left (C) and far right (O) of the diagram.
-
These AOs overlap to produce the MOs of CO in the middle. The MOs are either σ or π orbitals. They are bonding, nonbonding, or antibonding.
Conservation of Orbitals:
The overlap of two AOs produces two MOs, one bonding and one antibonding.
a. The overlap of two s AOs produces a σ MO and a σ* MO.
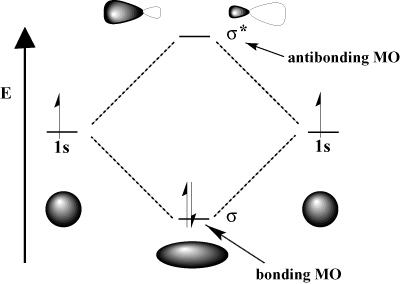
b. The end-on overlap of two p AOs produces a σ MO and a σ* MO.

c. The side-on overlap of two p AOs produces a π MO and a π* MO.
Interpreting the Diagram:
-
The 2s orbitals of C and O overlap to form the σ(2s) and σ*(2s) orbitals of CO.
-
The
#2p_z# orbitals of C and O overlap end-on to form the σ(2p) and σ*(2p) orbitals of CO. -
The
#2p_x# orbitals of C and O overlap side-on to form one set of π(2p) and π*(2p) orbitals for CO. -
The
#2p_y# orbitals of C and O overlap side-on to form a second set of π(2p) and π(2p) orbitals of CO. These two sets of orbitals are degenerate* (they have the same energy).
Adding the Electrons:
-
We place the 4 valence electrons of C in its AOs on the left hand side according to the Pauli Exclusion Principle and Hund's Rule.
-
We place the 6 valence electrons of O in its AOs on the right hand side according to the Pauli Exclusion Principle and Hund's Rule.
-
We place the 10 valence electrons in the 5 lowest MOs of CO.). Again we use the Pauli Exclusion Principle and Hund's Rule.
And we're done.


