An exothermic reaction is a reaction in which energy is given off, or, in other words, a reaction that has a #DeltaH<0# (see: enthalpy).
Here's a potential energy diagram for an exothermic reaction, the combustion of glucose
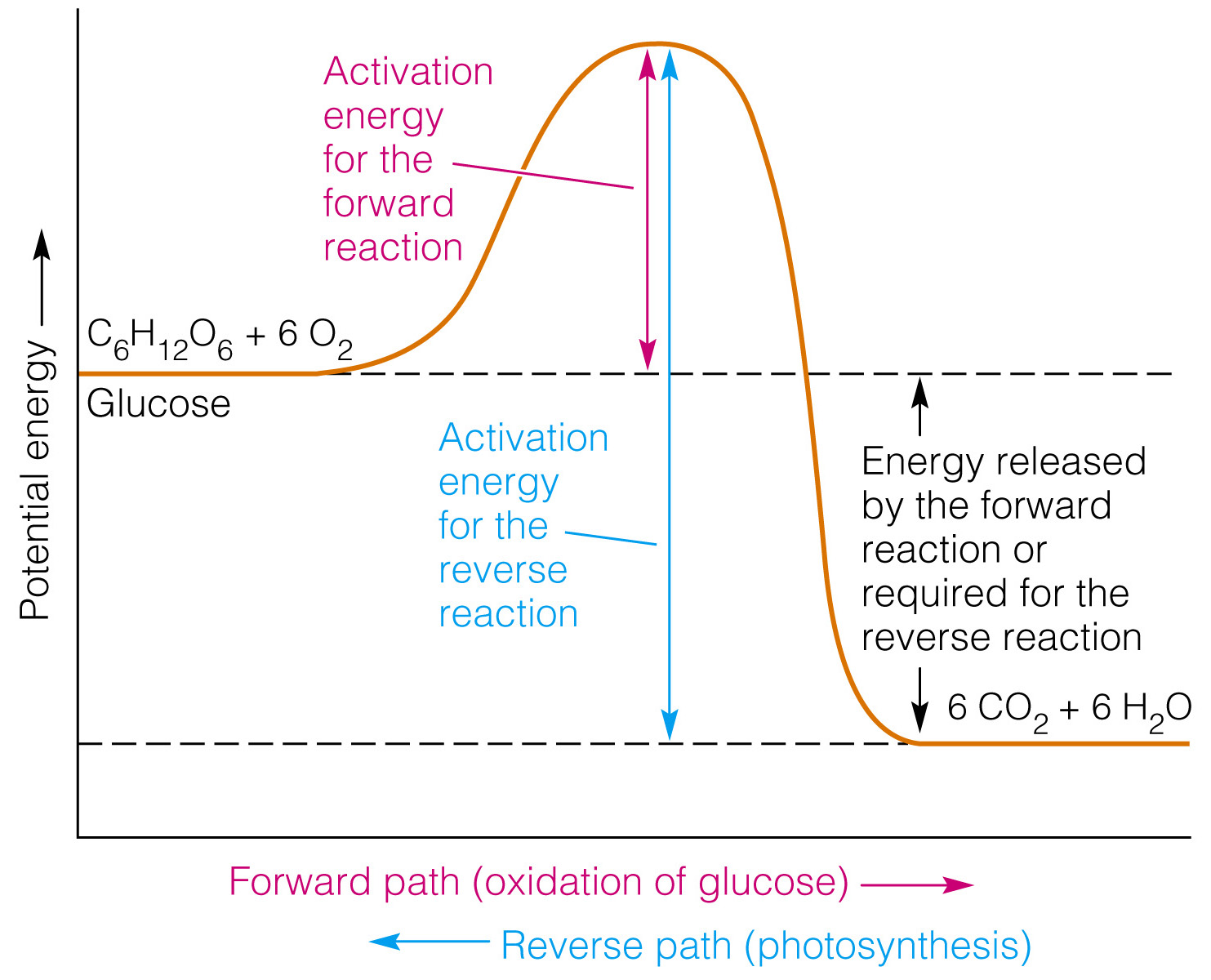
Notice that the potential energy of the reactants (#C_6H_12O_6 + 6O_2#) is smaller than the potential energy of the products (#6CO_2 + 6H_2O#); this difference in potential energy (on this diagram labeled as the energy released by the forward raection or required for the reverse reaction ) represents #DeltaH#.
We know that #DeltaH = H_(f) - H_i#, where
#H_f# represents the enthalpy (potential energy) of the products;
#H_i# represents the enthalpy (potential energy) of the reactans;
Since #H_f# is smaller than #H_i#. #DeltaH# will be smaller than zero (negative), a value that characterizes exothermic reactions.
So, let's say you would have to draw the diagram for the combustion of glucose. You'd start with the balanced chemical equation
#C_6H_12O_6 + 6O_2 -> 6CO_2 + 6H_2O#
Then you'd calculate #H_i# as the sum of the standard state enthalpies (#DeltaH_f^@# - usually given to you) of the reactans - each multiplied by their stoichiometric coefficients, and #H_f# as the sum of the standard state enthalpies of the products - again, each multiplied by their stoichiometric coefficients.
After this point, you'd just graph these values, along with #DeltaH = H_(f) - H_(i) <0# (in this case).

