How can you know if there is a doublet of doublets by looking at a structure?
2 Answers
Clearly, you must compare the proposed structure with its experimental
Explanation:
The point is that you do the experiment first; i.e. acquire the
No ONE method of analysis is sufficient for characterization. A new organic compound, before its existence can be accepted, must have (i)
So, to your question, how do you know that there is a doublet of doublets? Look at the spectrum first, and then try to rationalize that spectrum with a proposed structure.
Here's how I would do it.
Explanation:
A doublet of doublets (dd) is a pattern of four lines of approximately equal intensity that results from coupling to two different protons.

I can think of two situations in which I would expect to see dd splitting patterns:
- vinyl groups
- 1,3,4-trisubstituted benzenes
A. Vinyl groups
Typical coupling constants in alkenes are
Consider the
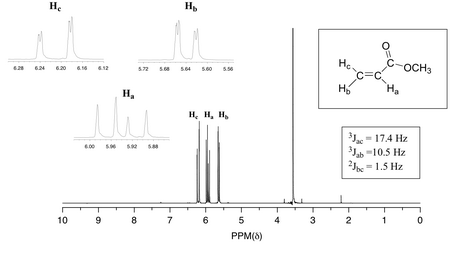
(From organic spectroscopy international)
Each proton on the vinyl group is split into a doublet of doublets by its neighbours.
1,3,4-Trisubstituted benzenes
Typical coupling constants in substituted benzenes are
Consider the
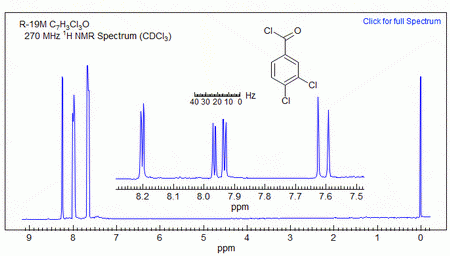
(From www.chem.wisc.edu)
The proton that has ortho and meta neighbours (on carbon 6) will be a doublet of doublets.
It appears at δ 7.95 (J = 8.5, 2.3 Hz).


