How did Bohr's model of the hydrogen atom differ from the previous model?
1 Answer
Bohr's model is known as the planetary model.
Rutherford found the nucleus with is gold foil experiment. His model of the atom had a small, dense, positively charged nucleus with the electrons all around the outside edge. In between the electrons and the nucleus was empty space.
Rutherford's model can be compared to a peach. The nucleus is the pit and the electrons are all found on the skin. The flesh of the peach is empty space. The electrons are equidistant from the nucleus.
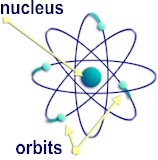
Diagram from http://www.daviddarling.info
Bohr's model has electrons at different energy levels around the nucleus. The electrons orbit the nucleus like the planets orbit the sun. Electrons can only be found in an energy level, never in between them.
Heat, electricity and light can excite the electrons. Electrons can absorb energy and jump to higher energy levels. The electrons can't hold the energy and fall back down to their ground state, emitting the energy as photons or bundles of light.
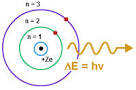
Diagram from TutorVista.com

