How did the "pi" bond help to explain the resonance in ozone?
1 Answer
Recall that resonance structures show different configurations of electron placement.
Explanation:
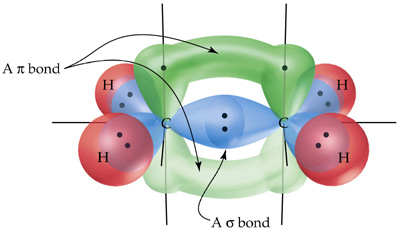
Also recall that a pi bond is an overlap of unhybridized p-orbitals as shown in the sp3 carbons in the image provided.
 wps.prenhall.com
wps.prenhall.com
Now, pi bonds are high energy and have a dispersed electron density making them weaker.
 https://commons.wikimedia.org
https://commons.wikimedia.org
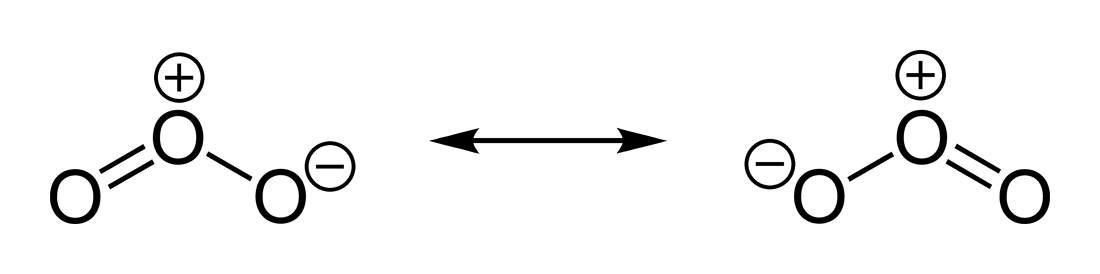
Note the difference between the two major resonance forms of ozone. The position of the double bond has changed as well as the formal charge on the terminal
Since pi bonds are higher energy than sigma bonds, they are more easily broken and may re-form elsewhere on the molecule. This movement of electrons between each resonance structure helps to delocalize any formal charges. The more resonance forms a structure has, the more stable the compound.
There are more valid resonance forms for

