How do chemical bonds hold substances together?
1 Answer
The modern chemical bond is conceived to be a region of high electron density between two positively charged nuclei, such that internuclear repulsion is minimized and a net attractive force results.
Explanation:
Ionic bonding is initially conceived to be the result of the transfer of electrons between species. On the other hand, simple covalent bonding is conceived to be the sharing of electrons between nuclei.
Consider this diagram depicting potential energy versus internuclear distance:
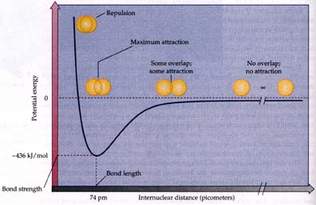
At infinite separation, there is no potential energy in the interaction of the 2 nuclei. At very close distances, the potential energy of the interaction becomes very high, because the positive nuclei overlap. However, there is demonstrably a distance where the potential energy of the interaction is at a minimum, and this internuclear distance is the BOND LENGTH. At this distance 2 electrons overlap to form a region of high electron density, an orbital, to which the positive nuclei are optimally attracted.

