How do E and Z isomers arise in molecules?
1 Answer
Dec 13, 2016
Structural isomerism derives from different connectivity for a given formula.
Explanation:
Geometric isomerism presumes the SAME structural isomerism, i.e. the same connectivity, however different geometry for the same structure. Organic chemistry provides rich examples of structural and geometric isomerism, and
Consider
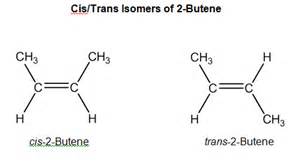
For both isomers, connectivity IS THE SAME.

