How do elements make up compounds?
2 Answers
Elements are substances composed of the same type of atom, for example oxygen (
Compounds are similar but are composed of atoms of different types. For example, nitrogen dioxide (
Compounds can be made in chemical reactions of elements. For example, during combustion of fuel and air in an engine, the gases get so hot that some oxygen molecules are dissociated to O atoms, and some nitrogen molecules are dissociated to N atoms. Some of these can recombine to form compounds like
Elements are composed of one kind of atom, while compounds are composed of two or more kinds of atoms that are chemically bonded.
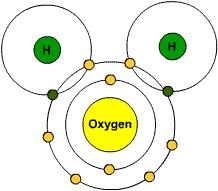
Atoms form compounds by forming either covalent or ionic bonds with atoms of other elements. A covalent bond occurs when atoms share valence electrons. For example, a water molecule (
In the diagram of the water molecule below, the overlapping areas are the covalent bonds formed when each hydrogen atom shares its valence electron (shown in green) with an oxygen valence electron (shown in yellow).
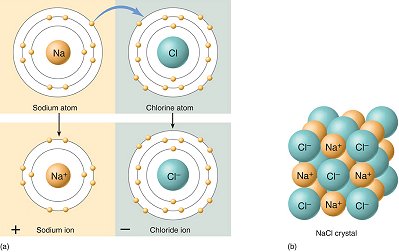
Ionic bonds form when an atom (usually a metal) transfers one or more electrons to another atom (usually a nonmetal). The metal atom becomes a positively charged ion and the nonmetal atom becomes a negatively charged ion. The electrostatic force of attraction between the oppositely charged ions forms the ionic bond.
The diagram below shows the transfer of an electron from a sodium atom to a chlorine atom, resulting in a positively charged sodium ion and a negatively charged chloride ion.