How do increases in temperature affect the density of ocean water?
1 Answer
And that is why the density increases with lowering temperature
Explanation:
Water is very abnormal substance because its density is lower in its solid state than its liquid state.
The density of water is 1kg/L(at 278.15K)
Density of ocean water also depends three factors
One is depth and air pressure, the other one is salinity of water and the other one is is temperature.
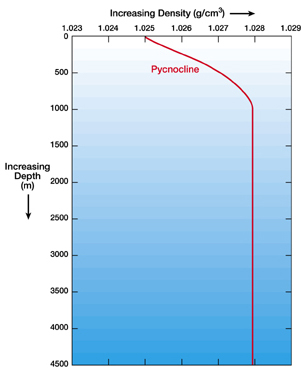
This graph shows the density of water increases with depth

This graph shows the density with altitude. This graph is completely different for almost all compounds because their density in the solid state is more than its density in liquid state. So density decreases with the altitude.Low pressure also creates the gas to expand and lose energy which cause it to lose energy. In atoms energy is actually heat which it loses.
You only need the original volume of to calculate the expansion of water in higher temperature.
Where
After you know the volume density can be easily calculated
Now look at this graph

You can see that the air pressure decreases with increasing altitude.So density of water decreases with decreasing air pressure.
Effect of Salinity on water

Now the effect of temperature on water

The graph is curved as when the water begins to freeze it looses density but increases again in low temperature but not near 32F
But the effect of heat on "sea water" is little different
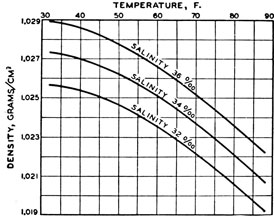
Because the water is evaporating and the salt is left which dissolves in the left water making it high concentration. This is just like heating a solution to get a more concentrated solution.
But still the rate of evaporation is less effective than the thermal expansion of water therefore at high temperature too the ocean water is still at lower density.
And that is why the density increases with lowering temperature

