How do you calculate the number of sigma pi bonds in #"H"_3"C-CH=CH-C≡CH"#?
1 Answer
Apr 8, 2016
Here's how you do it.
Explanation:
Every bond between two atoms contains 1 σ bond.
Every bond after the first bond is a π bond.
The expanded structural formula of
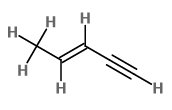
We count
The bond between
The bond between
Hence, pent-3-en-1-yne contains

