How do you calculate valence electrons for transition metals?
1 Answer
Valence electrons are only those in the outer (last) shell of an atom.
Explanation:
For transition metals this is no different from other atoms. What is sometimes confusing is that the highest numeric orbital with electrons is not always the valence orbital! See the following chart for the orbital filling sequence.
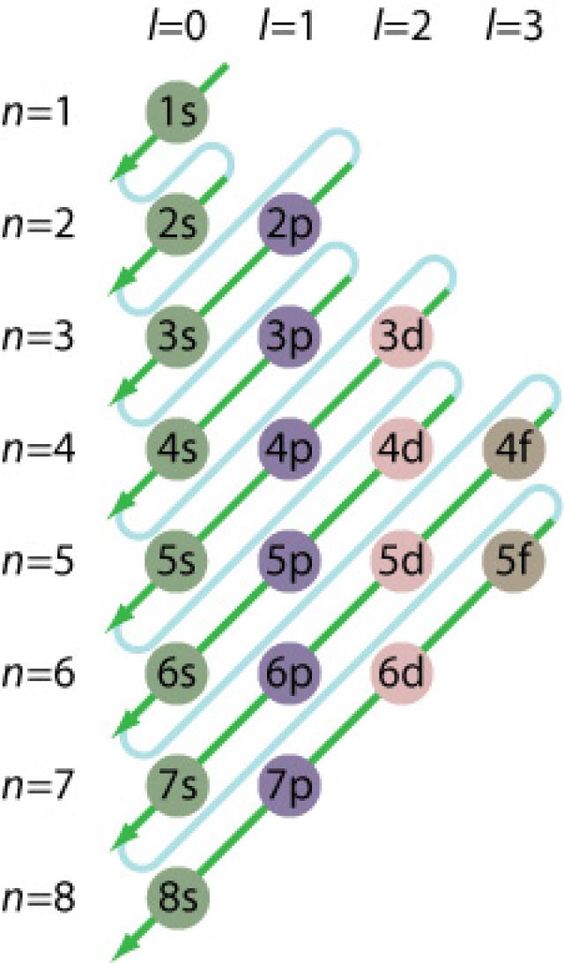
See also: http://chemistry.tutorcircle.com/inorganic-chemistry/electronic-configuration.html
And here: http://chemistry.tutorvista.com/inorganic-chemistry/electronic-configuration.html
The “short cut” using the Periodic Table is to look at the electron configuration of the previous Noble Gas (Group 18, or VIII in some tables) element. That will be the “core” electron configuration for the next Period, and you only need to add electrons into the next orbitals according to the diagram.
When all of your electrons are placed, the highest energy (not always highest number) orbital that is only partially filled is the valence orbital.

