How do you count sigma and pi bonds? For example, in the lewis structure azidothymidine there are 33 sigma and 5 pi bonds. How is this found?
1 Answer
Feb 9, 2016
Azidothymidine:
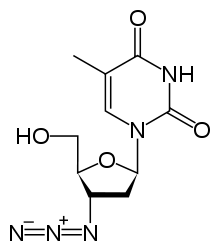
When you draw the purely skeletal structure, i.e. with no double or triple bonds, what is there counts as one sigma bond per single bond.
That gives you:
- Five
#"C"-"O"# #sigma# bonds - Six
#"C"-"N"# #sigma# bonds - Two
#"N"-"N"# #sigma# bonds - One
#"N"-"H"# #sigma# bond - One
#"O"-"H"# #sigma# bond - Seven
#"C"-"C"# #sigma# bonds
And don't forget the implicit hydrogens!
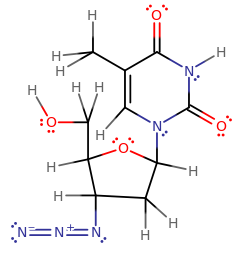
- Eleven
#"C"-"H"# #sigma# bonds
Now when you consider
- Two
#"N"-"N"# #pi# bonds - One
#"C"-"C"# #pi# bond - Two
#"C"="O"# #pi# bonds
So we have:
#5 + 6 + 2 + 1 + 1 + 7 + 11 = 33# #sigma# bonds#2 + 1 + 2 = 5# #pi# bonds

