How do you determine the number of pi and sigma bonds in a structure and locate them?
1 Answer
Using extended structure models
Explanation:
Here you have a more in-depth analysis of structure formulas.
Start off by writing down the respective compund using the extended structure model. Each simple covalent bond (represented by the line between 2 atoms) will be a sigma bond ;
Any other different from that ( double , triple covalent bonds ) will still have one sigma bond, the rest of the lines being counted as pi bonds
I'll give you an example:
Let's take the propane extended structure formula (which i stole from the answer above):
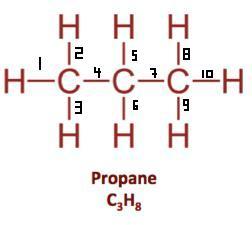
It is a saturated compound, so you will only have simple ( sigma ) covalent bonds - there are no pi bonds. Also, in this particular case ( for saturated aliphatic alkanes ) you can use a formula:
number of sigma bonds = number of atoms - 1 (which you can determine using the molecular formula )
If you would use that formula for propane, number of sigma bonds will be 3 + 8 - 1 = 10, as shown above.

