How do you determine the speed of a reaction?
1 Answer
The rate of a reaction can be measured in many different ways...
Explanation:
- You could measure the rate at which the products are formed.
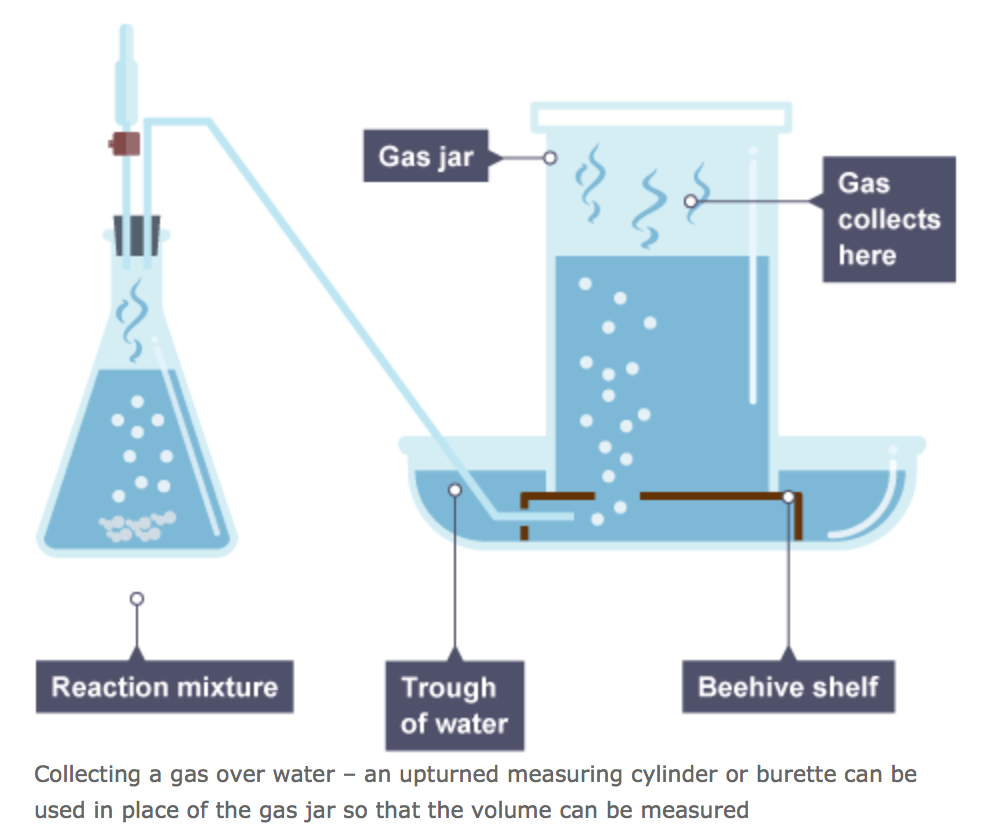
In the following reaction, where a metal reacts with an acid, hydrogen is produced. The hydrogen is a gas and therefore rises in water and moves towards the gas jar, where its volume may be measured. A reading of the volume may be taken every 10 seconds for example. When plotted, it will look something like this.
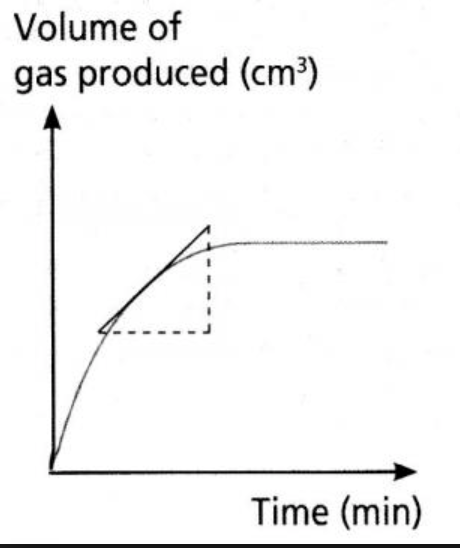
The rate of the reaction is different at every point on the graph. This is why it levels off. The gradient at a certain point is the rate of the reaction at that given time and may be calculated through finding the derivative of the function.
The reason why it levels off is that as the reactants are used up and the amount of products increases, it is more and more difficult for reactants to react.
Hope this helped! :D

