How do you draw #C_8H_18# (octane)? How many isomers are there? What are the names of the isomers?
1 Answer
Feb 28, 2016
Including stereoisomers, there are 24 stereoisomers of
Explanation:
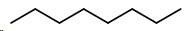
You work systematically. First, draw the straight-chain octane.
- Octane
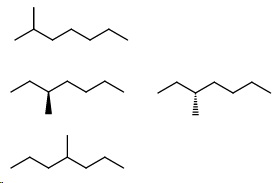
Next, draw all the heptanes. They will have a methyl group.
- 2-Methylheptane
- 3-Methylheptane (2 enantiomers)
- 4-Methylheptane
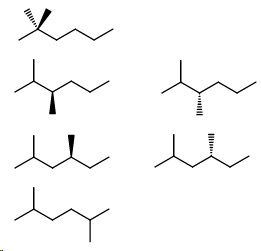
Next, come the hexane isomers. They will have either two methyl groups or an ethyl group.
All the isomers with a methyl group on carbon 2.
- 2,2-Dimethylhexane
- 2,3-Dimethylhexane (2 enantiomers)
- 2,4-Dimethylhexane (2 enantiomers)
- 2,5-Dimethylhexane
Then, all the isomers with a methyl group on carbon 3.
- 3,3-Dimethylhexane
- 3,4-Dimethylhexane (2 enantiomers + 1 meso compound)
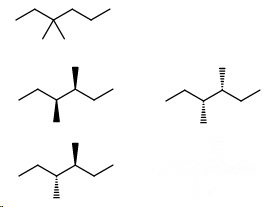
Then, the isomers with an ethyl group
- 3-Ethylhexane
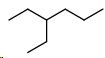
Now we draw the pentanes. They will have either three methyl groups or an ethyl and a methyl.
Isomers with three methyl groups. The possible patterns are 2,2,3, 2,2,4, 2,3,3 and 2,3,4.
- 2,2,3-Trimethylpentane (2 enantiomers)
- 2,2,4-Trimethylpentane (isooctane)
- 2,3,3-Trimethylpentane
- 2,3,4-Trimethylpentane
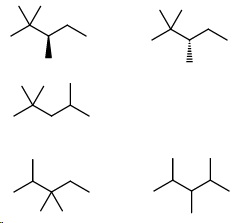
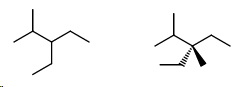
Then the ethylmethylpentanes.
- 3-Ethyl-2-methylpentane
- 3-Ethyl-3-methylpentane
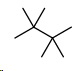
Finally, the butanes.
There is only one possibility.
- 2,2,3,3-Tetramethylbutane