How do you find chirality centers?
1 Answer
Chirality means a molecule that is mirrored won't be superimposable.
Explanation:
A chiral molecule can usually be found if there is no plane of symmetry, an example in every day life of this is your hands. (They are mirror images but one can't be put onto the other such that they would appear the same).
Picture of chiral hands:
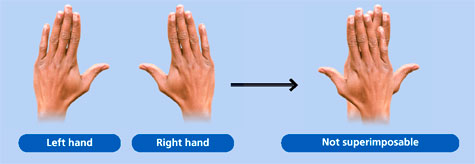
Image from: http://spicyip.com/2015/09/patent-office-rejects-tofacitinib-patent-application-an-analysis-part-i.html
To apply this to molecules one must first find a specific atom whether it be a Carbon or other, where there are four different substituents bonded to the atom.
Four different substituents bonded to a chirality center:

Image from: http://glossary.periodni.com/glossary.php?en=chiral+molecule
Sometimes it appears that the atom bonded directly to the atom of interest, as a chiral center, is the same as another one bonded to the atom of interest, so its necessary to look a bit further to see if substituent is indeed different from the other.
Example of this:
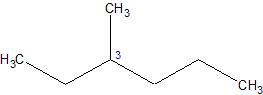
Image from: Created using ChemSketch 2015
One can see that the
Here's a video to help your understanding:
Video from: https://www.khanacademy.org/science/organic-chemistry/stereochemistry-topic/chirality-r-s-system/v/chirality-center-jay
Hope I helped :)

