What are chiral centers?
1 Answer
A chiral centre is an atom that has four different groups bonded to it in such a manner that it has a nonsuperimposable mirror image.
The term "chiral centre" has been replaced by the term chirality centre.
In the molecule below, the carbon atom is a chirality centre.
It has four different groups attached, and the two structures are nonsuperimposable mirror images of each other.
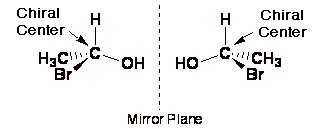
Molecules with a single chirality centre are chiral.
Molecules with more than one chirality centre are usually chiral. The exceptions are meso compounds.
For example, tartaric acid has two chirality centres, so you would expect it to have
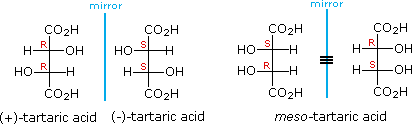
But there are only three isomers.
The (
The most common chirality centres in organic molecules are sp³ hybridized carbon atoms, because they can form four bonds.
Other types of chirality centre are
- quaternary N atoms

- tetravalent P atoms

Many nerve gases contain chiral P atoms.
- sulfoxides

In sulfoxides, the fourth group is a lone pair. Unlike in amines, the energy required to invert this stereocentre is high, so sulfoxides are optically stable.

