How do you name alkanes with halogens?
1 Answer
You name them as alkanes with the general prefix halo-.
Halogen substituents are functional groups. You can read how to name alkanes with functional groups at
http://socratic.org/questions/how-do-you-name-alkanes-with-functional-groups?source=search
The prefix names for the halogens are fluoro-, chloro-, bromo-, and iodo-. The general prefix is halo-.
Here's how it works.
1. Name the compound
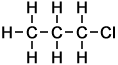
The compound has 3 C atoms, so it is a propane.
It contains a Cl atom, so it is a chloropropane.
The Cl is on C-1, so the name is 1-chloropropane.
2. Name the compound
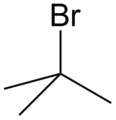
The longest continuous chain contains 3 C atoms, so it is a propane.
It contains a Br atom and a methyl group as substituents, so it is a bromomethylpropane.
The Br is on C-2, and the methyl group is on C-2. The name is 2-bromo-2-methylpropane.
3. Name the compound
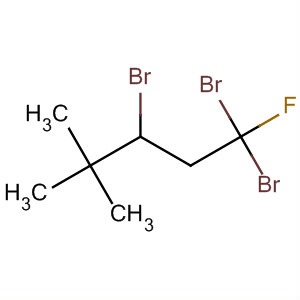
The longest continuous chain contains 5 C atoms, so it is a pentane.
It contains 3 Br atoms, a fluorine atom, and 3 methyl groups on the main chain, so it is a trifluorobromotrimethylpentane.
We number from the right hand end. The Br atoms are on C-1 and C-2; the F atom is on C-1; and the methyl groups are on C-4.
The name is 1,1,2-tribromo-1-fluoro-4,4-dimethylpropane.
Note:
1. Commas separate numbers from numbers.
2. Hyphens separate numbers from letters.
3. The substituents are listed in alphabetical order: bromo < fluoro < methyl.
4. The multiplying prefixes (di-, tri, etc.) do not determine the alphabetical order.

