How do you write a balanced nuclear equation for alpha decay of Po-218?
1 Answer
Here's how you can do that.
Explanation:
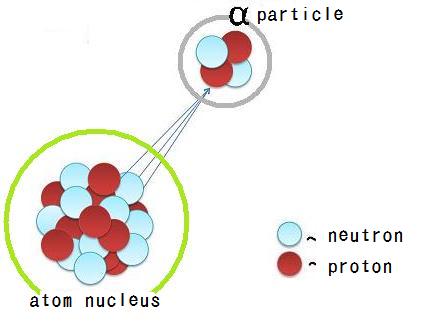
When a radioactive nuclide undergoes alpha decay, it emits an alpha particle,
This means that after the alpha particle is emitted
- the mass number of the nuclide will decrease by
#4# #-># this happens because the alpha particle contains#2# protons and#2# neutrons- the atomic number of the nuclide will decrease by
#2# #-># this happens because the alpha particle contains#2# protons
You can thus say that you have
#""_ (color(white)(.)84)^218"Po" -> ""_ (color(white)(.)(84-2))^((218 - 4))"X" + ""_ 2^4alpha#
A quick look in the Periodic Table of Elements will show that the element that has the atomic number equal to
#84 - 2 = 82 -># conservation of charge
is lead,
#218 - 4 = 214 -># conservation of mass
The balanced nuclear equation that describes the alpha decay of polonium-218 will look like this
#""_ (color(white)(.)84)^218"Po" -> ""_ (color(white)(.)82)^214"X" + ""_ 2^4alpha#
As