How does electronegativity affect the strength of an acid?
1 Answer
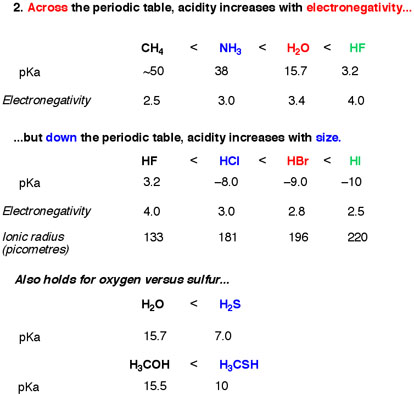
Two almost similar factors work behind this : 1. Across a row in periodic table, acidity increases with the increase in electronegativity. But as we go 2. down a group the periodic table, acidity increases with the decrease of electronegativity.
Explanation:
The first point makes pretty sense. But the second one is the confusing one. A reason behind this phenomena is the small size of elements in the top of a group of periodic table. As we go down a group, size of atom increases. So what's the problem with the smaller atoms? The problem is, their bond length is short too. So it is hard for them to dissociate in water, which doesn't seem to be a problem for the larger atoms down the group.
Another reason is the stability of the ion. For example, HF is an acid, it dissociates in water and gives a proton. In this process,