What is an example to how hybridization affects the equilibrium the strength of an acid?
1 Answer
Aug 8, 2015
The most striking example is the acidity of alkynes.
Explanation:
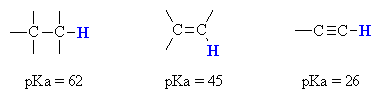
(from www.chem.ucalgary.ca)
-
An alkane
#"C-H"# bond is#sp^3# hybridized and has 25 %#s# character. -
An alkene
#"C-H"# bond is#sp^2# hybridized and has 33 %#s# character. -
An alkyne
#"C-H"# bond is#sp# hybridized and has 50 %#s# character.
The
The change in hybridization from

