Factors Determining Strength
Key Questions
-
Answer:
Well, take the hydrogen halides....
Explanation:
#HF, HCl, HBr, HI#
#stackrelrarr"increasing acid strength"# And we examine the reaction....
#HX(aq) + H_2O(l) rightleftharpoons H_3O^+ + X^-# The stronger the acid, the farther to the right the equilibrium moves...
While entropy CERTAINLY influences the reaction (i.e. fluoride anion is entropically disfavoured), we could certainly point to the role of enthalpy in this reaction. The LARGER halides possess POORER orbital overlap with respect to the small hydrogen atom. And acidic strength INCREASES for the higher hydrogen halides.
-
Answer:
The more charged ions produced, the stronger the acid. It all depends on how well the acid ionizes.
Explanation:
When an acid dissolves in water (to form an acidic solution), the hydrogen atoms present in all acids ionise. The extent of how much they ionize determines the strength. A solution in which all (or almost all) the atoms ionize will be a strong acid, whereas one where only a few ions ionize will be a weak acid. However, this is not the same as concentration, as it refers to the concentration of only the hydrogen ions e.g. if there are two acids both with the same concentration, the one with the most ionized hydrogen atoms will be the strongest (and therefore have the lowest pH).
So the more charged ions produced, the stronger the acid - and the lower the pH.
I hope this helps, let me know if you need anymore information - or something explained further:)
-
Answer:
The factors that affect the strength of a carboxylic acid are
- Resonance stabilization
- Electron donating groups
- Electron withdrawing groups
Explanation:
Resonance Stabilization
The ethanoate ion is strongly stabilized by two equivalent resonance structures.
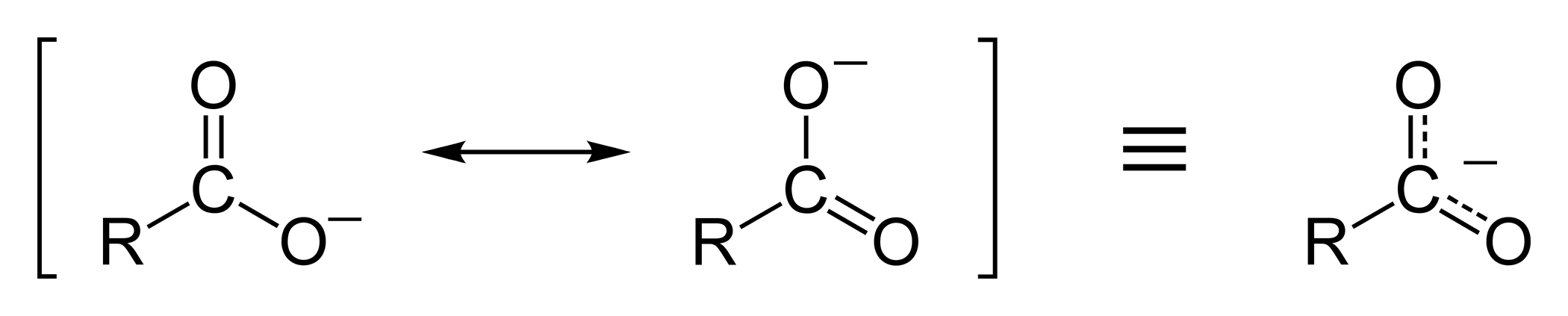
The phenoxide ion is less effectively stabilized.

In three contributors, the negative charge is delocalized into the ring. These are not major contributors because (a) they disrupt the cyclic π system in the ring and (b) O is more electronegative than C.
The ethoxide ion has no resonance contributors.
Electron Donating Groups
Electron donating groups decrease the acidity of an organic acid.
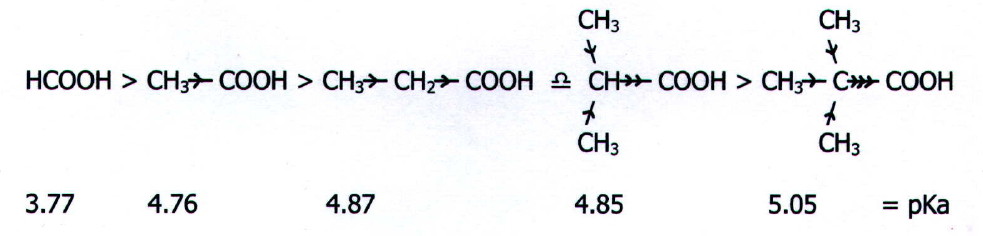
Alkyl groups are electron donating. They tend to "push" electrons away from themselves.
This increases the electron density in the O-H bond. The H atom is more strongly attracted to the O. It is less likely to leave, so the acid is weaker.
Electron Withdrawing Groups
Electron withdrawing groups increase the acidity of an organic acid.

An electronegative atom like Cl can pull electron density toward itself.
This decreases the electron density in the O-H bond. The H atom is less strongly attracted to the O. It is more likely to leave, so the acid is stronger. The more Cl atoms you attach, the stronger the acid becomes.