How does IR spectroscopy work?
1 Answer
Infrared (IR) spectroscopy uses infrared radiation to excite the molecules of a compound and generates an infrared spectrum of the energy absorbed by a molecule as a function of the frequency or wavelength of light.
Different types of bonds respond to the IR radiation differently. For example, triple and double bonds are shorter and stiffer than single bonds, and therefore will vibrate at higher frequencies. The types of atoms involved in the bonds are also relevant. For example, O-H bonds are stronger than C-H bonds, so O-H bonds vibrate at higher frequencies. IR spectroscopy therefore allows us to identify the various functional groups present in a compound.
It is worth noting that a dipole moment is required for a molecule to absorb IR radiation. In general, if a bond has a dipole moment, its stretching frequency causes an absorption in the IR spectrum. If a bond is symmetrically substituted and has zero dipole moment, its stretching vibration is weak or entirely absent in the spectrum (IR-active vs. IR-inactive vibrations). However, bonds with zero dipole moments sometimes produce weak absorptions because molecular collisions, rotations, and vibrations make them unsymmetrical part of the time.
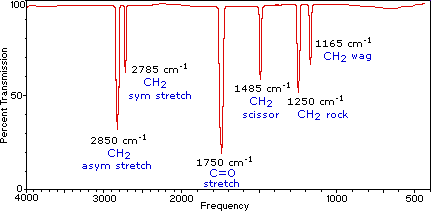
Here is an example of an IR spectrum for formaldehyde.