How does one nucleus affect other nuclei in NMR?
1 Answer
The magnetic field of one nucleus affects the field experienced by nearby nuclei.
Consider the
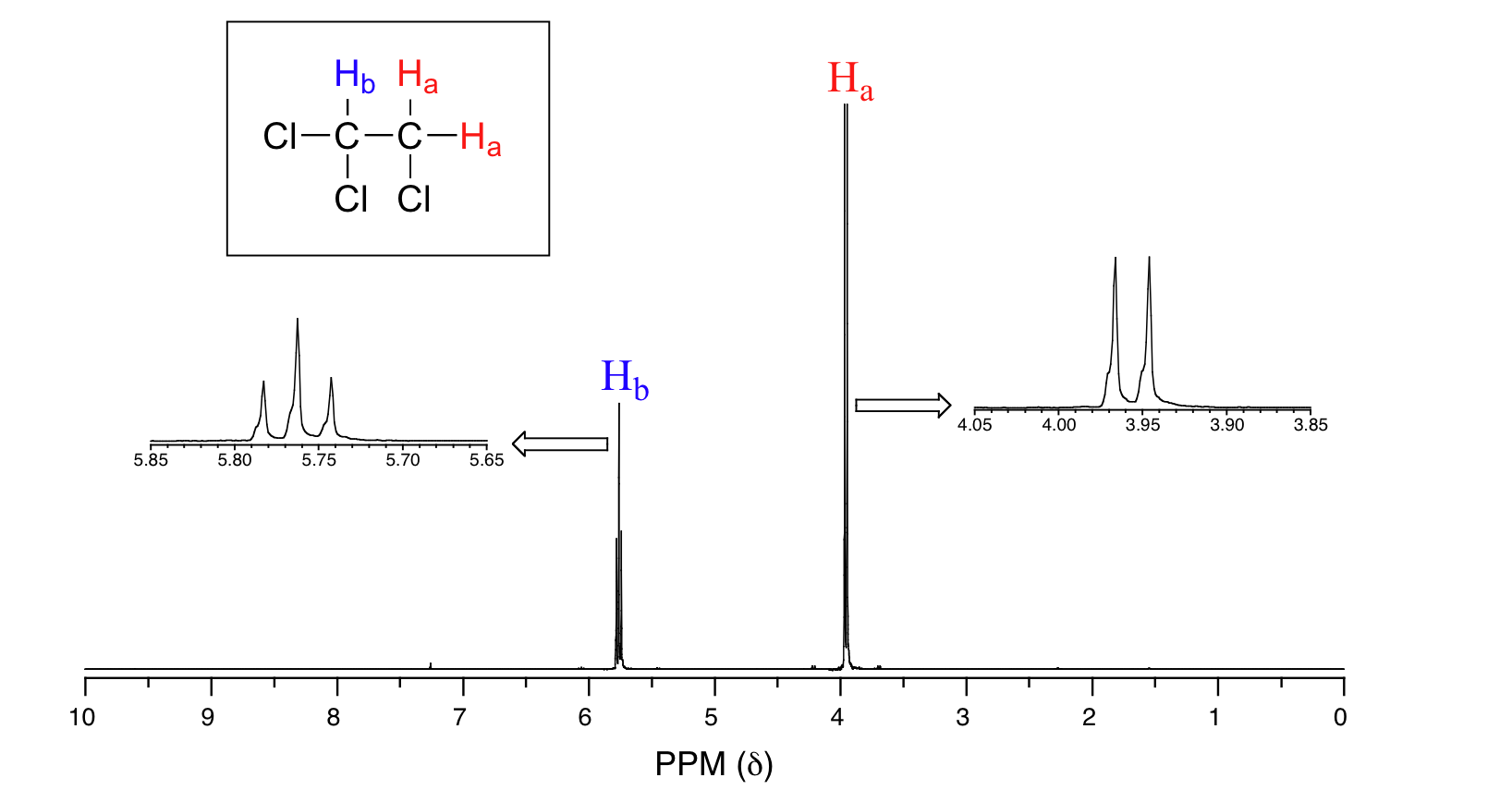
It consists of a 2H signal at δ 3.9 (
The CH₂ signal is a doublet because the H atom in the CH next door has a small magnetic field of its own.
This field could be aligned with the external magnetic field or opposed to it.
Depending on the way it is aligned, it will either strengthen or weaken the field felt by the CH₂ hydrogens.
There is a 50:50 chance of each arrangement, so there will be two peaks due to the CH₂ hydrogens. They will be close together and have equal areas.
The CH signal is a triplet because that hydrogen is experiencing any one of three slightly different magnetic fields.
The hydrogens on the next door CH₂ group have four possible magnetic arrangements.
Two of them cancel each other, so the CH hydrogen could feel three possible magnetic fields. This gives three peaks close together — a triplet.
The areas under the peaks are in the ratio of 1:2:1, because that represents the probabilities of the three arrangements.
We can extend this argument to other numbers of adjacent H atoms.
Here’s a video on NMR splitting patterns.

