What are the coupling constants (#J#)?
1 Answer
The coupling constant
For example:
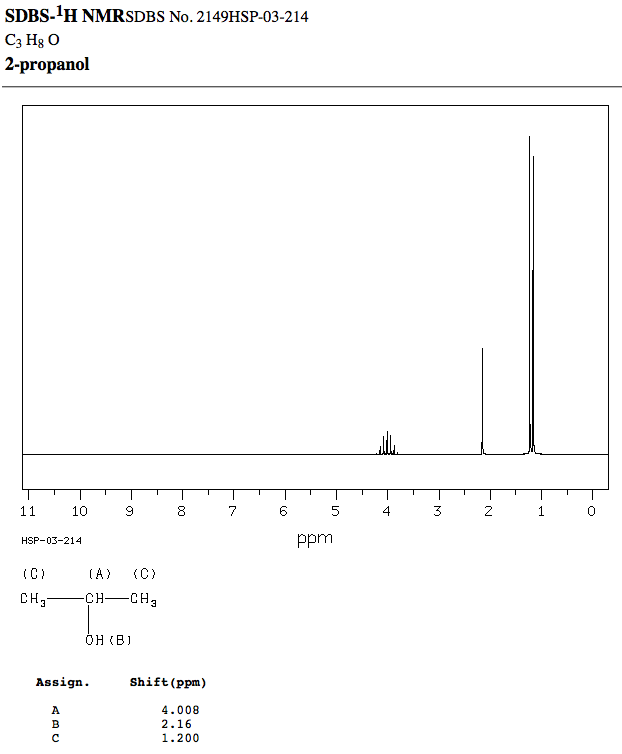
Peak Data:
#"Hz"" "" " "" " ppm"" ""Intensity"# Proton A:
#"371.56"" ""4.149"" ""24"#
#"365.38"" ""4.080"" ""56"#
#"359.25"" ""4.012"" ""72"#
#"353.13"" ""3.943"" ""60"#
#"347.06"" ""3.876"" ""28"# Proton B:
#"193.00"" ""2.155"" ""335"# Proton C:
#"110.44"" ""1.234"" ""1000"#
#"108.19"" ""1.209"" ""27"#
#"104.31"" ""1.165"" ""939"#
#"102.06"" ""1.140"" ""25"#
What is shown here for proton A is that
1
#-># 1-1#-># 1-2-1#-># 1-3-3-1#-># 1-4-6-4-1
It is not visible in this zoom, but the distance between each peak is roughly identical. This distance is the numerical equivalent of the coupling constant
#4.149 - 4.080 = "0.069 ppm"#
#4.080 - 4.012 = "0.068 ppm"#
#4.012 - 3.943 = "0.069 ppm"#
#3.943 - 3.876 = "0.067 ppm"#
Interestingly enough, if you look at protons C at the averaged
#1.234 - 1.165 = "0.068 ppm"#
From the identical (or nearly-identical) coupling constant, you can determine which protons are "communicating" with each other and thus which protons they neighbor.
If you take this number and multiply it by the
#"0.068 ppm" * "89.56 MHz"#
# = 0.068 ("Hz")/("MHz") * "89.56 MHz"#
#= color(blue)"6.09 Hz"#
Indeed, for proton C,
Therefore, without seeing the structure of the analyzed molecule, you can still figure out that proton A and protons C are coupling/"communicating" with each other.

