How does sp2 hybridized carbon differ from sp3?
1 Answer
Nov 11, 2016
Here are the differences I see.
Explanation:
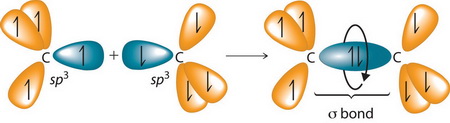
An
(From wps.prenhall.com)
It can react with another
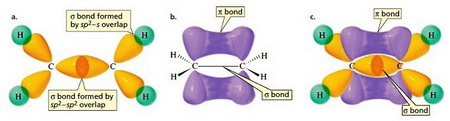
(From www.kshitij-iitjee.com)
An
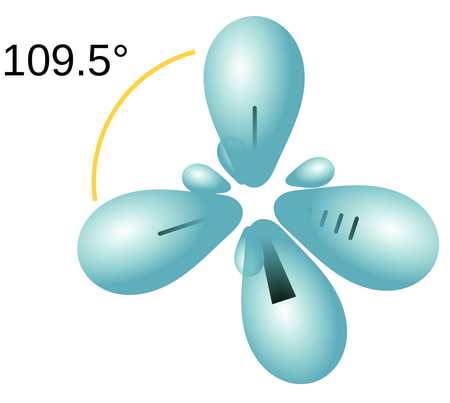
(From BC Open Textbooks)
It can form only σ bonds with other atoms.