How does the polarization of the carbonate ion make the thermal decomposition of CaCO3 more likely?
1 Answer
I think I see the confusion. You may not be seeing the intramolecular effects. The decomposition reaction is:
#"CaCO"_3(s) stackrel(Delta" ")(->) "CaO"(s) + "CO"_2(g)#
The effect of interest is the polarization towards the
Yes, there is an ion-pairing attraction going on, but that is not going against the ability of the
Since the calcium cation is highly-positively-charged, and is somewhat small, one might call it a "hard acid" (from Hard-Soft Acid/Base Theory), because it can capably concentrate negative charge density towards itself, and we call that great polarizing ability. We can also say that
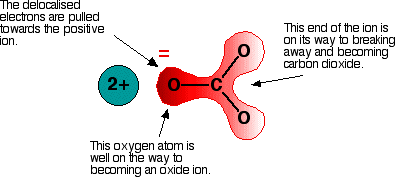
Thus, it pulls electron density from the
That bond will be broken upon heating in order to perform the decomposition reaction.

