How does the strength of a covalent bond relate to its length?
1 Answer
Oct 5, 2014
The shorter the covalent bond, the stronger it is.
Covalent bonds can either be single, double or triple.
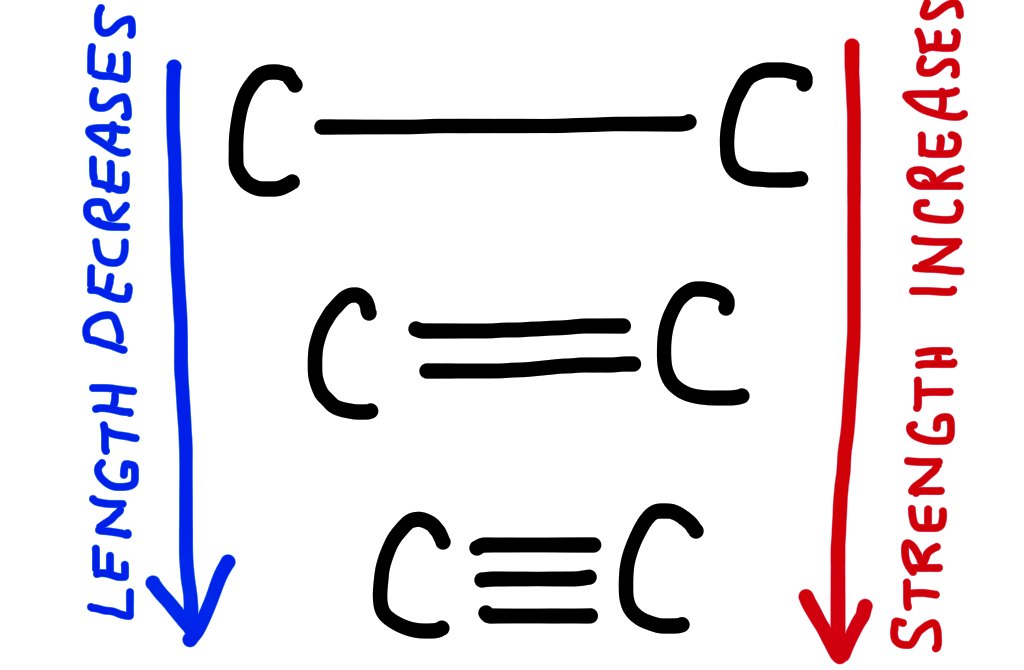
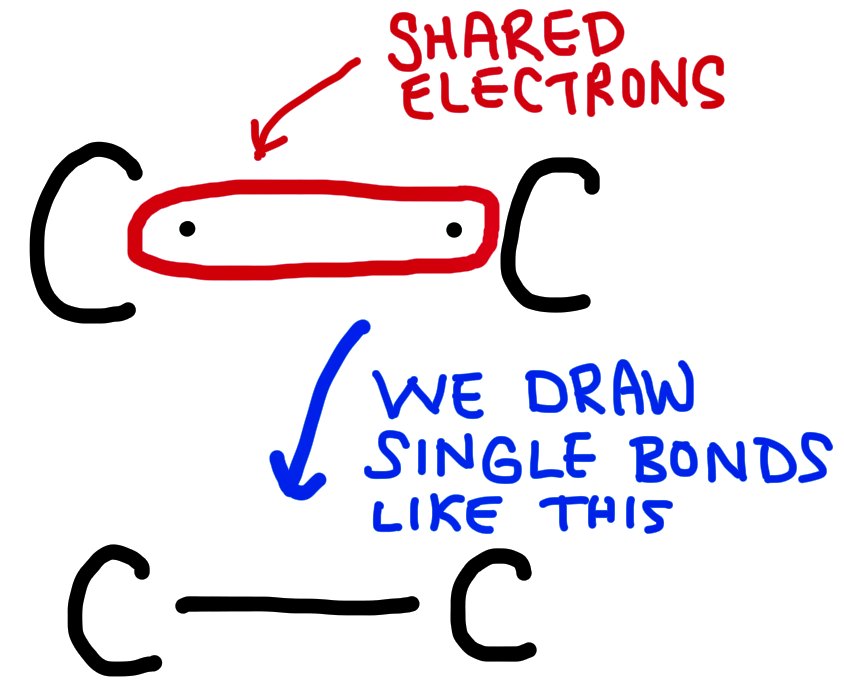
A single bond involves 2 electrons, shared between two atoms and is the longest/weakest.
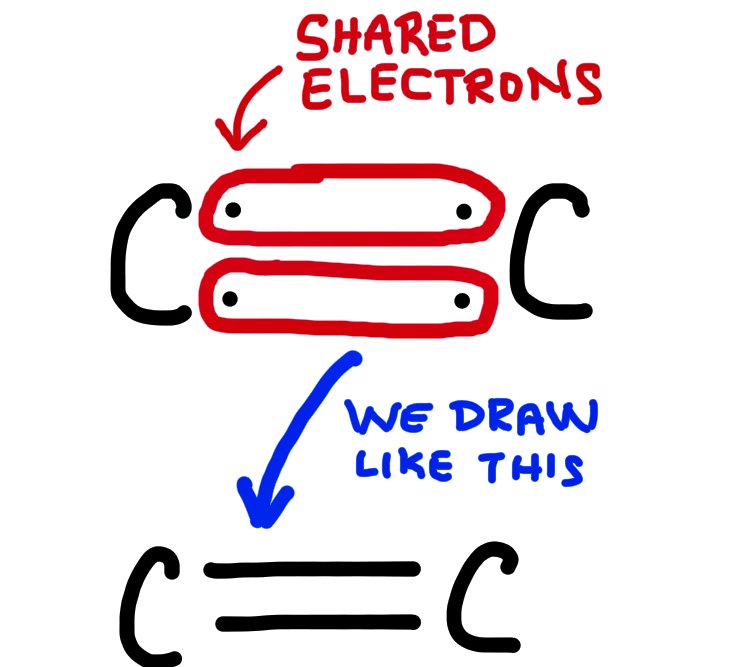
A double bond involves 4 electrons, shared between 2 atoms and is shorter but stronger than a single bond.
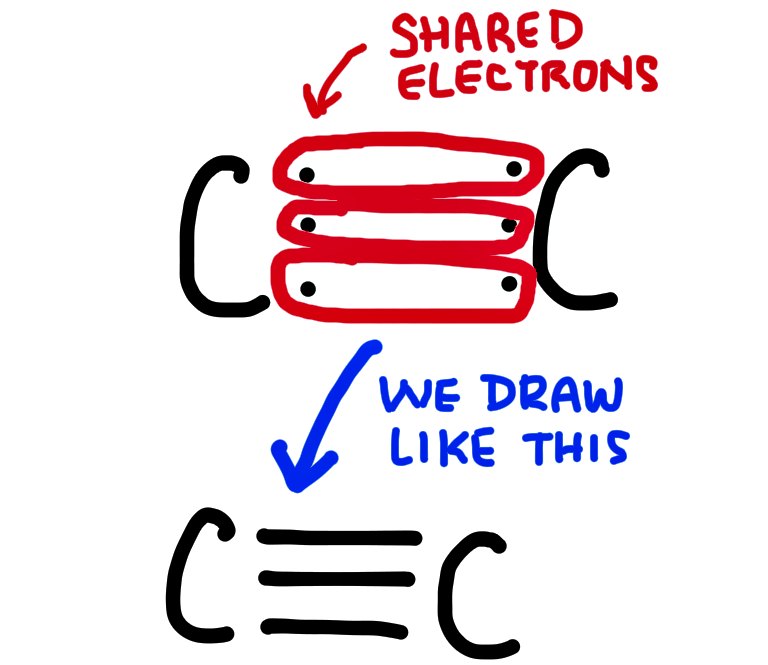
A triple bond involves 6 electrons, shared between 2 atoms and is the shortest and strongest.
Learn more here:

